Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.54E: Consider the following solutions: Sodium chloride s in water Sucrose s in water C20H42(s) in...
Related questions
Question
I'm having trouble figuring out the last three:
Ions exhibiting significant Brønsted acid characteristics
Ions exhibiting significant Brønsted base characteristics
Ions exhibiting no significant Brønsted acid or base characteristics
What do I need to do?
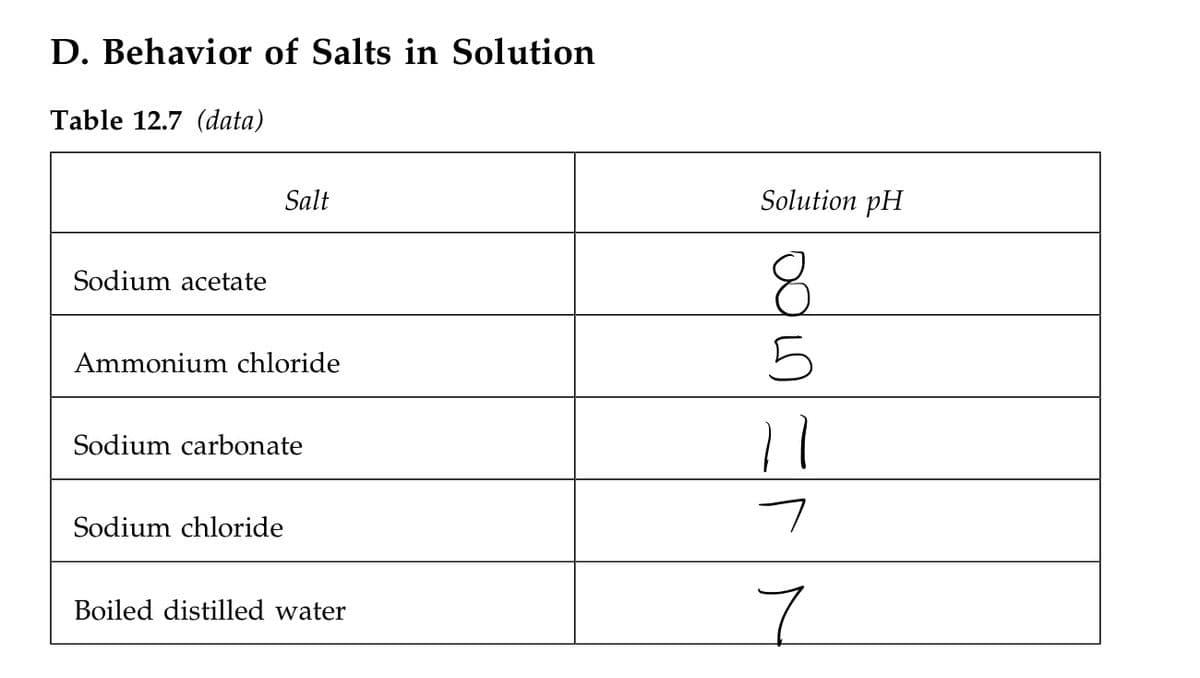
Transcribed Image Text:D. Behavior of Salts in Solution
Table 12.7 (data)
Salt
Solution pH
8.
Sodium acetate
Ammonium chloride
Sodium carbonate
Sodium chloride
7.
Boiled distilled water
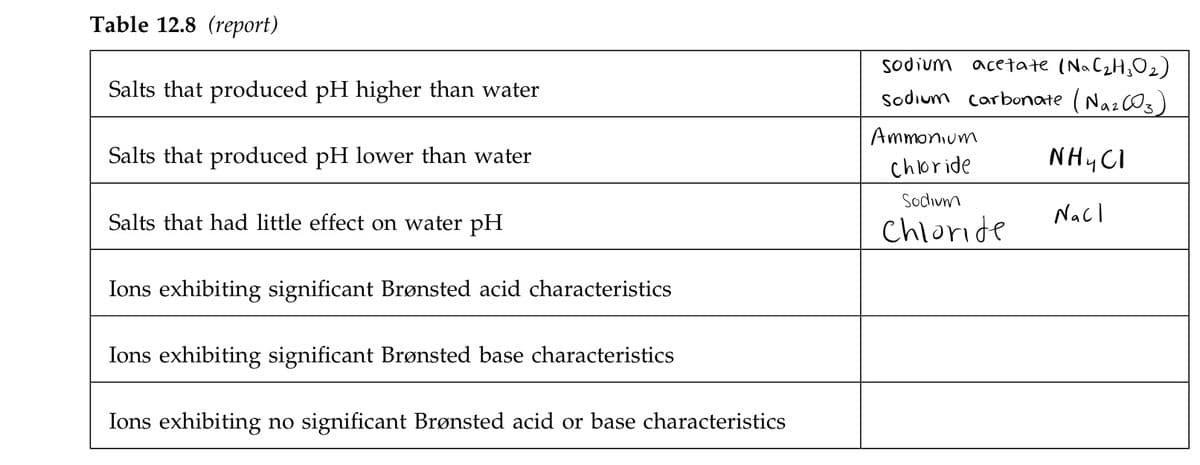
Transcribed Image Text:Table 12.8 (report)
sodium acetate (NaC2H;O2)
Sodium carbonate (NA2CO3)
Salts that produced pH higher than water
Ammonium
Salts that produced pH lower than water
chloride
NH CI
Sodivm
Salts that had little effect on water pH
Chloride
Nacl
Ions exhibiting significant Brønsted acid characteristics
Ions exhibiting significant Brønsted base characteristics
Ions exhibiting no significant Brønsted acid or base characteristics
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,