Data: Standard molar enthalpy of formation of H20 (g): AfH°1 = -241.6 kJ mol- Standard molar enthalpy of formation of H2O2 (g): AfH°2 = -140.4 kJ mol-! Standard molar enthalpies of bond dissociation: AdissH°(H2(g)) = 435.6 kJ mol·'; AdissH°(O2(g) = 497.8 kJ mol·l Calculate:
Data: Standard molar enthalpy of formation of H20 (g): AfH°1 = -241.6 kJ mol- Standard molar enthalpy of formation of H2O2 (g): AfH°2 = -140.4 kJ mol-! Standard molar enthalpies of bond dissociation: AdissH°(H2(g)) = 435.6 kJ mol·'; AdissH°(O2(g) = 497.8 kJ mol·l Calculate:
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 101QRT: Appendix J lists standard molar entropies S, not standard entropies of formation rS. Why is this...
Related questions
Question
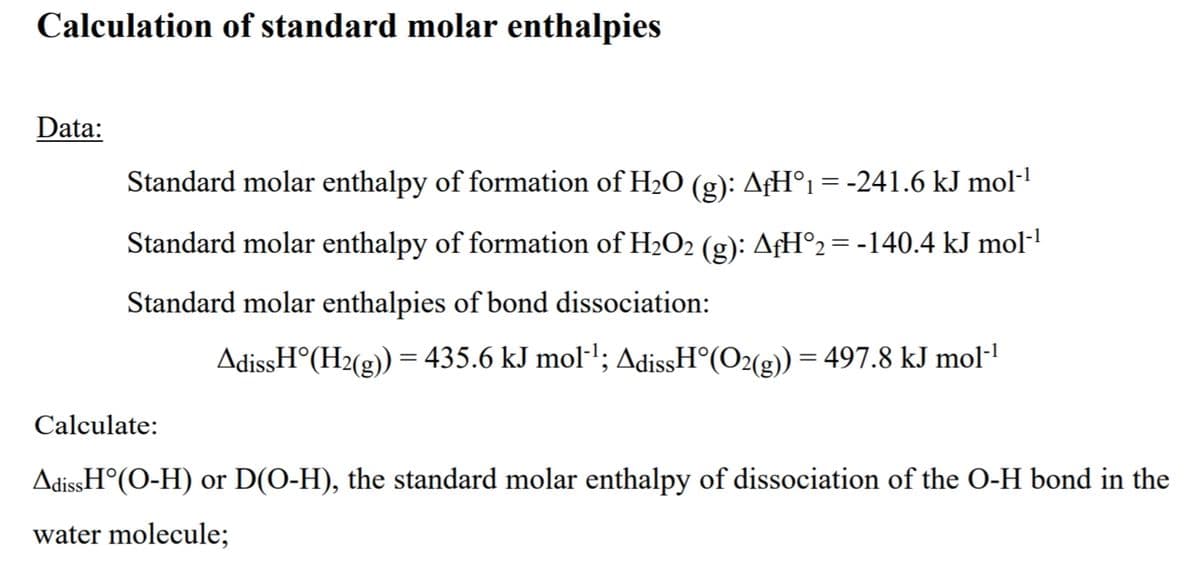
Transcribed Image Text:Calculation of standard molar enthalpies
Data:
Standard molar enthalpy of formation of H2O (g): AfH°1 = -241.6 kJ mol·1
Standard molar enthalpy of formation of H2O2 (g): AfH°2= -140.4 kJ mol-'
Standard molar enthalpies of bond dissociation:
AdissH°(H2(g)) = 435.6 kJ mol·'; AdissH°(O2(g)) = 497.8 kJ mol·"
Calculate:
AdissH°(O-H) or D(O-H), the standard molar enthalpy of dissociation of the O-H bond in the
water molecule;
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax