Standard molar enthalpy of formation of H2O (g): AfH°1 = -241.6 kJ mol Standard molar enthalpy of formation of H2O2 (g): AfH°2= -140.4 kJ mol- %3D Standard molar enthalpies of bond dissociation: AdissH°(H2(g)) = 435.6 kJ mol·'; AdissH°(O2(g)) = 497.8 kJ mol·" %3D Calculate: AdissH°(O-O) or D(O-O), the standard molar enthalpy of dissociation of the O-O bond in the molecule of hydrogen peroxide H2O2 of structural formula H-O-O-H. The dissociation
Standard molar enthalpy of formation of H2O (g): AfH°1 = -241.6 kJ mol Standard molar enthalpy of formation of H2O2 (g): AfH°2= -140.4 kJ mol- %3D Standard molar enthalpies of bond dissociation: AdissH°(H2(g)) = 435.6 kJ mol·'; AdissH°(O2(g)) = 497.8 kJ mol·" %3D Calculate: AdissH°(O-O) or D(O-O), the standard molar enthalpy of dissociation of the O-O bond in the molecule of hydrogen peroxide H2O2 of structural formula H-O-O-H. The dissociation
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section: Chapter Questions
Problem 96QRT
Related questions
Question
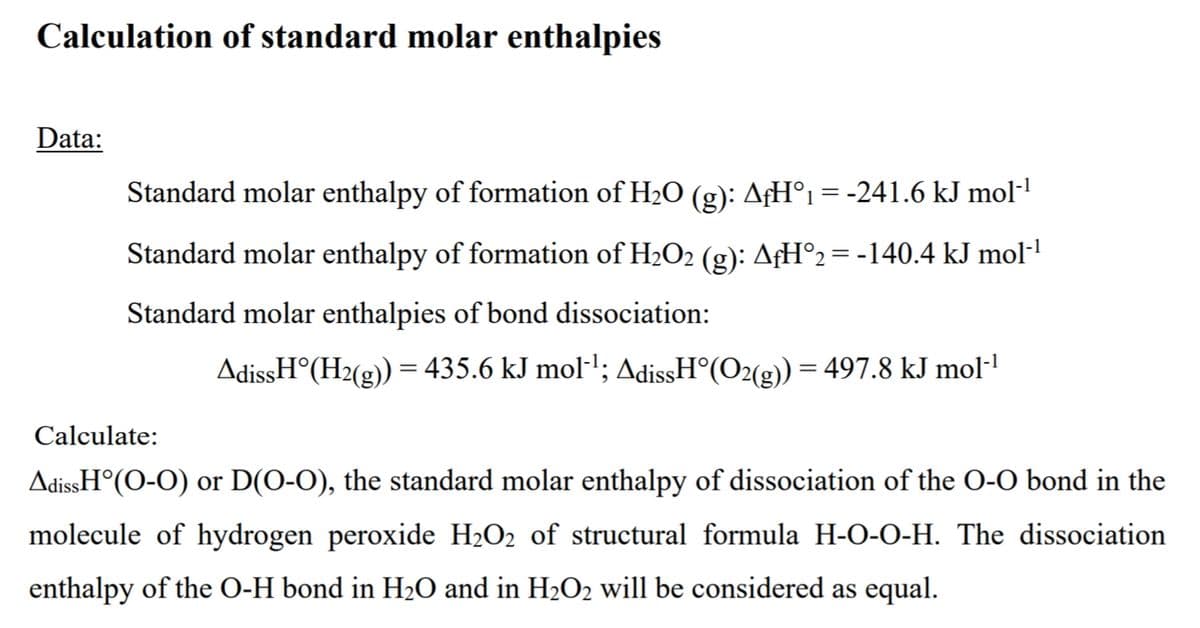
Transcribed Image Text:Calculation of standard molar enthalpies
Data:
Standard molar enthalpy of formation of H2O (g): AƒH°1 = -241.6 kJ mol-1
Standard molar enthalpy of formation of H2O2 (g): AfH°2= -140.4 kJ mol-'
Standard molar enthalpies of bond dissociation:
AdissH°(H2(g)) = 435.6 kJ mol-'; AdissH°(O2(g)) = 497.8 kJ mol-!
Calculate:
AdissH°(O-O) or D(O-O), the standard molar enthalpy of dissociation of the O-O bond in the
molecule of hydrogen peroxide H2O2 of structural formula H-O-0-H. The dissociation
enthalpy of the O-H bond in H2O and in H2O2 will be considered as equal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
