Data Table 1: NaHCO, Reaction Data Mass of evaporating dish (g) 47.19919 Mass of evaporating dish + NaHCO: (g) 47.B0029 Mass of NaHCO: (g) a30119 Mass of evaporating dish + NaCl product after 1s heating (g) Mass of evaporating dish + NaCl product after 2nd heating (g) Mass of evaporating dish + NaCl product after 3rd heating (g) (if necessary) 47.04L09 47.0469 Mass of NaCl product (g) (actual yield) 020559
Data Table 1: NaHCO, Reaction Data Mass of evaporating dish (g) 47.19919 Mass of evaporating dish + NaHCO: (g) 47.B0029 Mass of NaHCO: (g) a30119 Mass of evaporating dish + NaCl product after 1s heating (g) Mass of evaporating dish + NaCl product after 2nd heating (g) Mass of evaporating dish + NaCl product after 3rd heating (g) (if necessary) 47.04L09 47.0469 Mass of NaCl product (g) (actual yield) 020559
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 63QAP: Butane gas, C4H10, is sold to campers as bottled fuel. Its density at 25C and 1.00 atm is 2.38 g/L....
Related questions
Question

Transcribed Image Text:2. Determine the limiting reactant, theoretical yield and percent yield of NaCl in the NaHCO; reaction.
Show your work for each step.
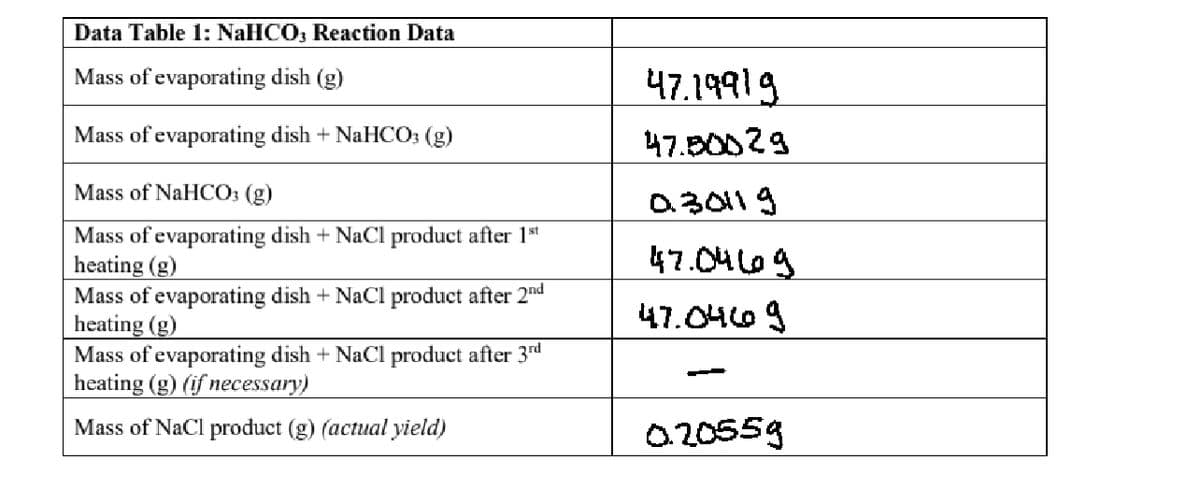
Transcribed Image Text:Data Table 1: NaHCO, Reaction Data
Mass of evaporating dish (g)
47.19919
Mass of evaporating dish + NaHCO: (g)
47.B0029
Mass of NaHCO: (g)
a30119
Mass of evaporating dish + NaCl product after 1st
heating (g)
Mass of evaporating dish + NaCl product after 2nd
heating (g)
Mass of evaporating dish + NaCl product after 3rd
heating (g) (if necessary)
47.04L69
47.0469
Mass of NaCl product (g) (actual yield)
020559
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning