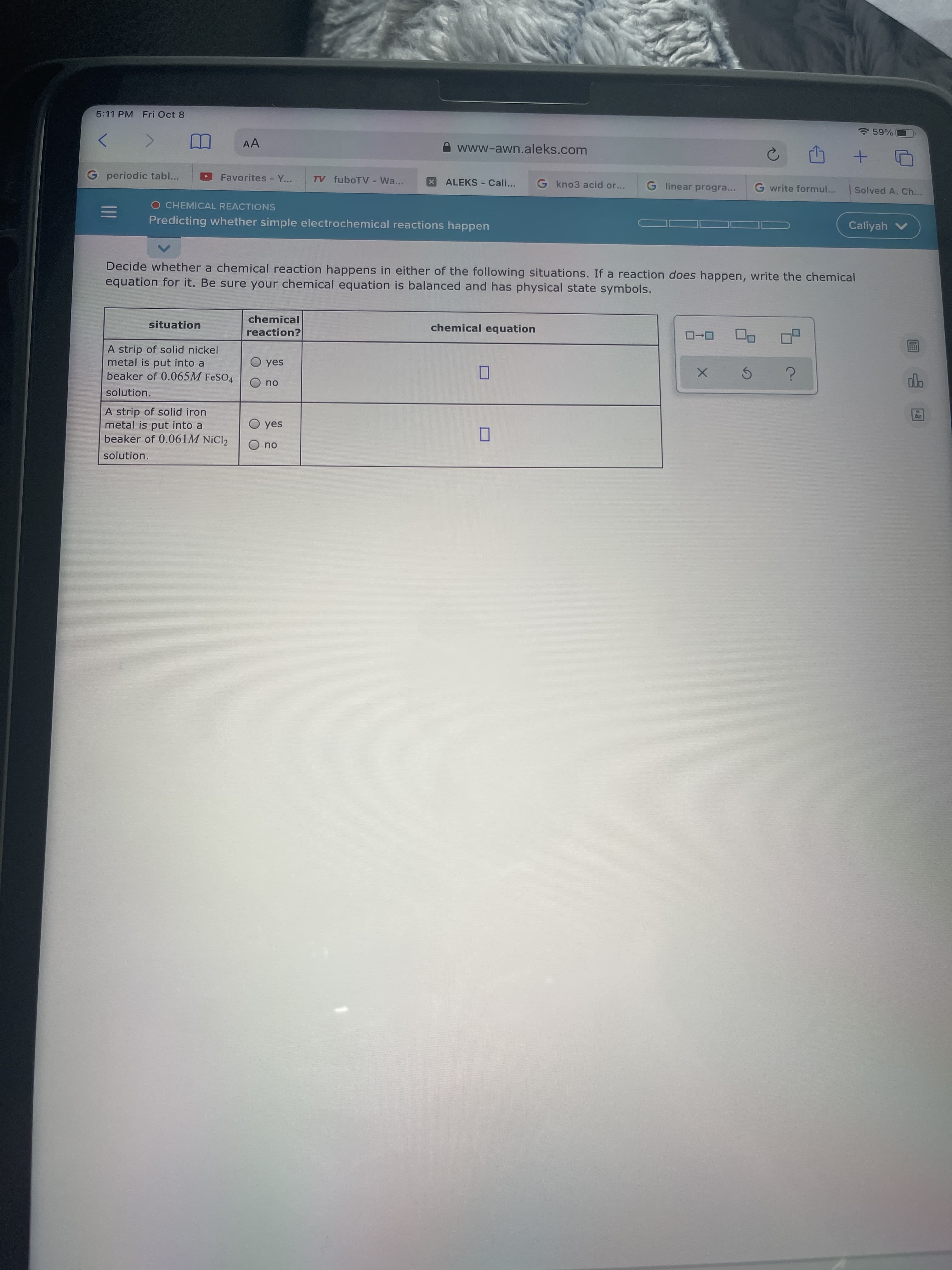
Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. chemical situation reaction? chemical equation ローロ A strip of solid nickel metal is put into a beaker of 0.065M FeSO4 O yes no solution. A strip of solid iron metal is put into a beaker of 0.061M NiCl2 yes no solution.
Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. chemical situation reaction? chemical equation ローロ A strip of solid nickel metal is put into a beaker of 0.065M FeSO4 O yes no solution. A strip of solid iron metal is put into a beaker of 0.061M NiCl2 yes no solution.
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.29QAP
Related questions
Question
Transcribed Image Text:5:11 PM Fri Oct 8
59%
AA
www-awn.aleks.com
G periodic tabl...
Favorites - Y...
TV fuboTV Wa...
ALEKS - Cali...
G kno3 acid or...
G linear progra...
G write formul...
Solved A. Ch...
O CHEMICAL REACTIONS
Caliyah v
Predicting whether simple electrochemical reactions happen
Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical
equation for it. Be sure your chemical equation is balanced and has physical state symbols.
chemical
situation
chemical equation
reaction?
A strip of solid nickel
metal is put into a
beaker of 0.065M FeSO4
O yes
ou
solution.
A strip of solid iron
metal is put into a
beaker of 0.061M NiCl2
yes
ou
solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,