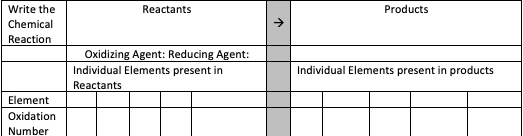
Balance Equation: 2Al + 3CuCl2 * 2H2O -> 2Cu + 2AlCl3 + 6H20 1. Reproduce the following table in your document. Write the chemical reaction again in the indicated spot, typing the reactants under the word “reactants” and products under the word “products”. Then in the row labeled “Element”, list each element in the reactants and products. Assign oxidation numbers to each element in the reactants and products and write the oxidation number underneath each element in the table below. Explain how these were derived. (see table) 2. From the reactants, identify the oxidizing agent and reducing agent and input this into the table above. Explain, in a few sentences, how you determined the difference between the two. 3. Type out the complete ionic equation and the net ionic equation for the reaction. Explain how you arrived at your answer in a narrative (3-5 sentences), including why you canceled any ions as spectators.
Balance Equation: 2Al + 3CuCl2 * 2H2O -> 2Cu + 2AlCl3 + 6H20
1. Reproduce the following table in your document. Write the
(see table)
2. From the reactants, identify the oxidizing agent and reducing agent and input this into the table above. Explain, in a few sentences, how you determined the difference between the two.
3. Type out the complete ionic equation and the net ionic equation for the reaction. Explain how you arrived at your answer in a narrative (3-5 sentences), including why you canceled any ions as spectators.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps






