Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. situation A strip of solid nickel metal is put into a beaker of 0.068M AgNO3 solution. A strip of solid silver metal is put into a beaker of 0.083M Ni(NO3)2 solution. chemical reaction? yes O no Oyes Ono chemical equation 0 X 2 3 c
Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. situation A strip of solid nickel metal is put into a beaker of 0.068M AgNO3 solution. A strip of solid silver metal is put into a beaker of 0.083M Ni(NO3)2 solution. chemical reaction? yes O no Oyes Ono chemical equation 0 X 2 3 c
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU6: Showtime: Reversible Reactions And Chemical Equilibrium
SectionU6.2: How Backward: Reversible Reactions
Problem 7E
Related questions
Question
100%
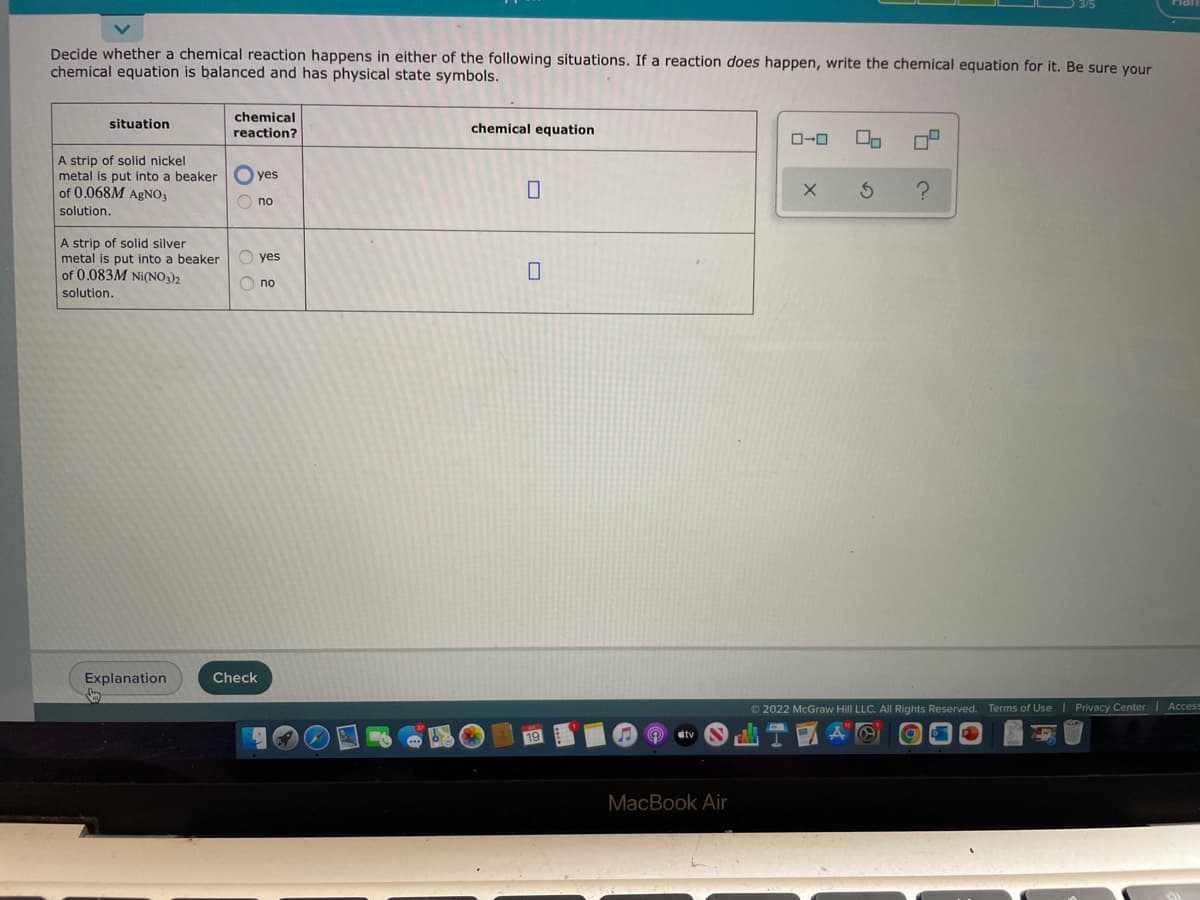
Transcribed Image Text:Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your
chemical equation is balanced and has physical state symbols.
situation
A strip of solid nickel
metal is put into a beaker
of 0.068M AgNO3
solution.
A strip of solid silver
metal is put into a beaker
of 0.083M Ni(NO3)2
solution.
Explanation
chemical
reaction?
yes
Ono
yes
O no
Check
1
chemical equation
0
#tv
MacBook Air
ローロ
X
2
00
S ?
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Access
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning