Define Electrolyte. 1. Solutions that are electrolytes have ions in solution. These ions are surrounded by layers of water molecules known as the solvation sphere. Ions with their solvation spheres are able to move freely about the solution and thus can be capable of 2. an electrical charge. For each of the following compounds identify the transporting ions that will occur in solution. Name Cation(s) Anion(s) Compound +2 (aq) Bа barium chloride 2 Cl (aq) ВаClz Na2S ZnBr2 FeCl3 LIOH AGC2H3O2 Mn(CIO4)2 К,РОД CuCl2 Al(NO3)s Co2(SO4)3 81
Define Electrolyte. 1. Solutions that are electrolytes have ions in solution. These ions are surrounded by layers of water molecules known as the solvation sphere. Ions with their solvation spheres are able to move freely about the solution and thus can be capable of 2. an electrical charge. For each of the following compounds identify the transporting ions that will occur in solution. Name Cation(s) Anion(s) Compound +2 (aq) Bа barium chloride 2 Cl (aq) ВаClz Na2S ZnBr2 FeCl3 LIOH AGC2H3O2 Mn(CIO4)2 К,РОД CuCl2 Al(NO3)s Co2(SO4)3 81
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 7CR: efine the term strong electrolyte. What types of substances tend to be strong electrolytes? What...
Related questions
Question
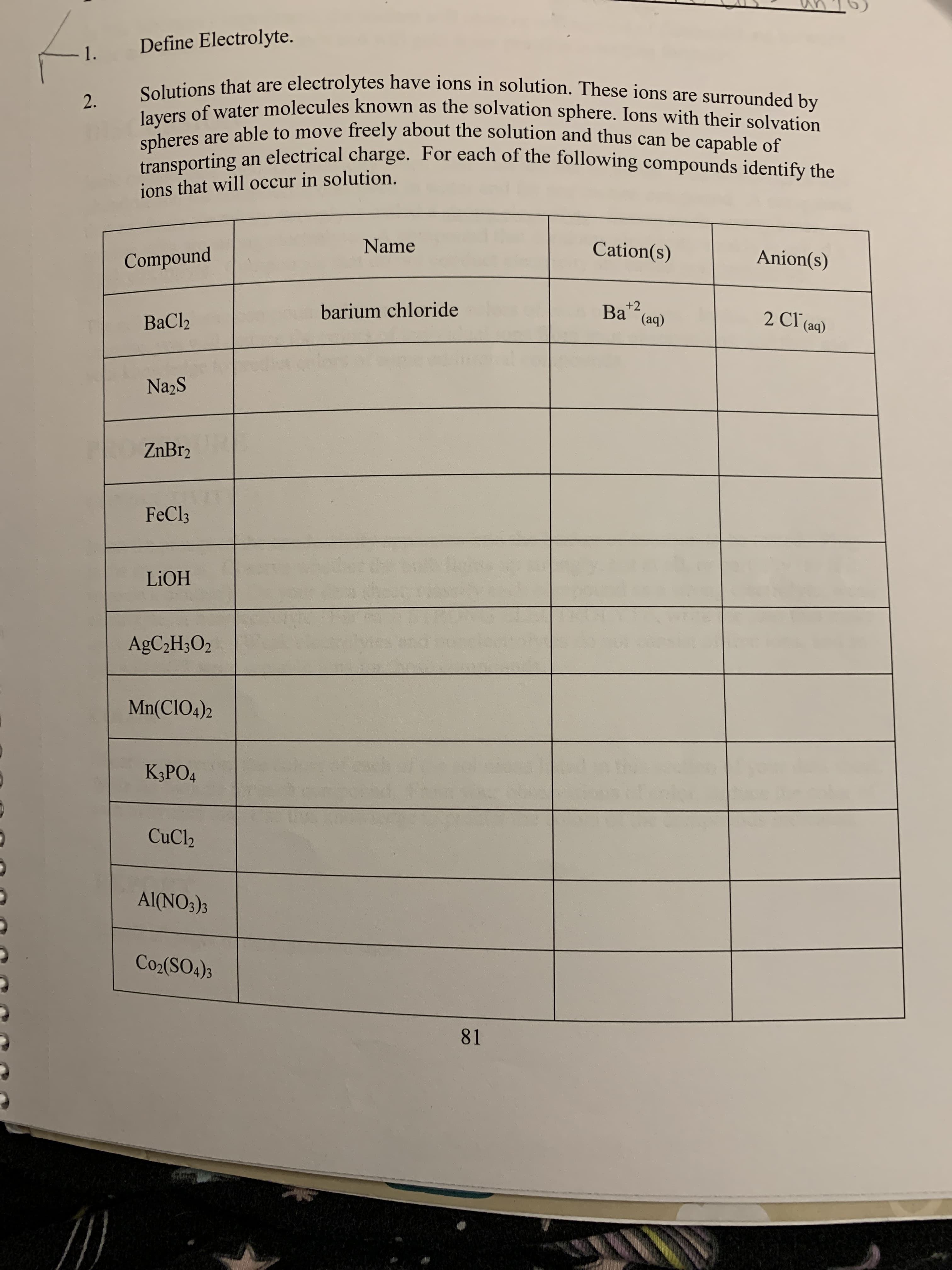
Transcribed Image Text:Define Electrolyte.
1.
Solutions that are electrolytes have ions in solution. These ions are surrounded by
layers of water molecules known as the solvation sphere. Ions with their solvation
spheres are able to move freely about the solution and thus can be capable of
2.
an electrical charge. For each of the following compounds identify the
transporting
ions that will occur in solution.
Name
Cation(s)
Anion(s)
Compound
+2
(aq)
Bа
barium chloride
2 Cl (aq)
ВаClz
Na2S
ZnBr2
FeCl3
LIOH
AGC2H3O2
Mn(CIO4)2
К,РОД
CuCl2
Al(NO3)s
Co2(SO4)3
81
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning