der the peaks for pentafluorobenzene and benzene in the gas chromatogram shown here. The elution time for unretained solute is 1.06 min. The open tubular column is 30.0 m in length and 0.530 mm in diameter, with a layer of stationary phase 3.0 μm thick on the inner wall. a) Measruing the width, w, at the baseline on the chromatogram, find the number of plates for these two compounds b) Use your answer to (a) to find the resolution between the two peaks c) Using the number of plates N=sqrtN1*N2 with the values from (a) calcuate what the resolution should be and compare your answer with the measured resolution in b
der the peaks for pentafluorobenzene and benzene in the gas chromatogram shown here. The elution time for unretained solute is 1.06 min. The open tubular column is 30.0 m in length and 0.530 mm in diameter, with a layer of stationary phase 3.0 μm thick on the inner wall. a) Measruing the width, w, at the baseline on the chromatogram, find the number of plates for these two compounds b) Use your answer to (a) to find the resolution between the two peaks c) Using the number of plates N=sqrtN1*N2 with the values from (a) calcuate what the resolution should be and compare your answer with the measured resolution in b
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 78E: Compare the functions of homogeneous and heterogeneous catalysts.
Related questions
Question
4.Consider the peaks for pentafluorobenzene and benzene in the gas chromatogram shown here. The elution time for unretained solute is 1.06 min. The open tubular column is 30.0 m in length and 0.530 mm in diameter, with a layer of stationary phase 3.0 μm thick on the inner wall.
a) Measruing the width, w, at the baseline on the chromatogram, find the number of plates for these two compounds
b) Use your answer to (a) to find the resolution between the two peaks
c) Using the number of plates N=sqrtN1*N2 with the values from (a) calcuate what the resolution should be and compare your answer with the measured resolution in b
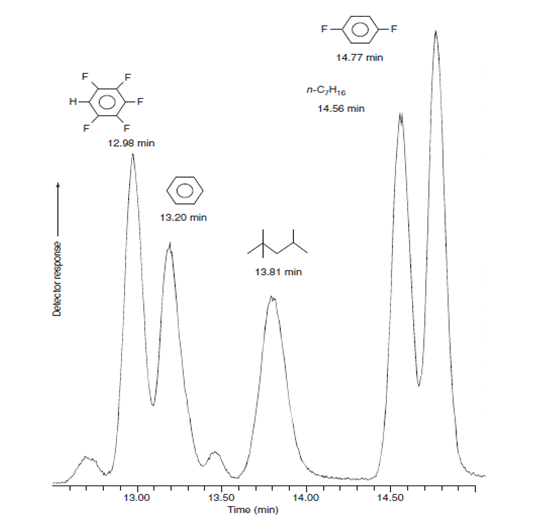
Transcribed Image Text:14.77 min
F
n-C,H,6
H-
14.56 min
12.98 min
13.20 min
13.81 min
13.50
Timo (min)
13.00
14.00
14.50
LL
eSuodsa JOLag
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning