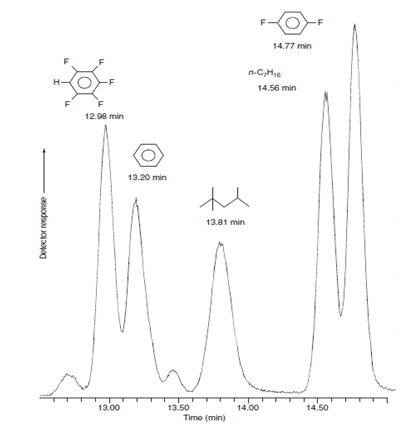
1) Consider the peaks for pentafluorobenzene and benzene in the gas chromatogram shown here. The elution time for unretained solute is 1.06 min. The open tubular column is 30.0 m in length and 0.530 mm in diameter, with a layer of stationary phase 3.0 μm thick on the inner wall. a) Find the adjusted rentention times and rentention factors for both compounds. b) Find the relative rentention c) Measuring the w1/2 on the chromatrogram , find the number of plates, N1 and N2, and the plate height for these two compounds.
1) Consider the peaks for pentafluorobenzene and benzene in the gas chromatogram shown here. The elution time for unretained solute is 1.06 min. The open tubular column is 30.0 m in length and 0.530 mm in diameter, with a layer of stationary phase 3.0 μm thick on the inner wall. a) Find the adjusted rentention times and rentention factors for both compounds. b) Find the relative rentention c) Measuring the w1/2 on the chromatrogram , find the number of plates, N1 and N2, and the plate height for these two compounds.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 96AE: Iodomethane (CH3I) is a commonly used reagent in organic chemistry. When used properly, this reagent...
Related questions
Question
1) Consider the peaks for pentafluorobenzene and benzene in the gas chromatogram shown here. The elution time for unretained solute is 1.06 min. The open tubular column is 30.0 m in length and 0.530 mm in diameter, with a layer of stationary phase 3.0 μm thick on the inner wall.
a) Find the adjusted rentention times and rentention factors for both compounds.
b) Find the relative rentention
c) Measuring the w1/2 on the chromatrogram , find the number of plates, N1 and N2, and the plate height for these two compounds.
Transcribed Image Text:14.77 min
n-C,Hie
14.56 min
12.98 min
13.20 min
13.81 min
13.00
13.50
Time (min)
14.00
14.50
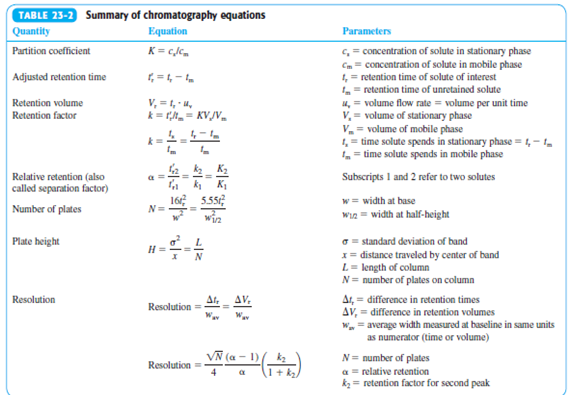
Transcribed Image Text:TABLE 23-2 Summary of chromatography equations
| Quantity
Partition coefficient
Equation
Parameters
K= cJc.
c, = concentration of solute in stationary phase
Cm = concentration of solute in mobile phase
1, = retention time of solute of interest
= retention time of unretained solute
4, = volume flow rate = volume per unit time
V, = volume of stationary phase
V = volume of mobile phase
1, = time solute spends in stationary phase = 1,- 1.
, = time solute spends in mobile phase
Adjusted retention time
Retention volume
Retention factor
V, =1, 4,
k = r.- KV/Vm
Relative retention (also
a =
Subscripts I and 2 refer to two solutes
called separation factor)
w = width at base
win = width at half-height
16
5.55
Number of plates
N=
win
Plate height
G = standard deviation of band
H =
x= distance traveled by center of band
L = length of column
N= number of plates on column
N
Ar,
AV.
Ar, = difference in retention times
AV, = difference in retention volumes
W = average width measured at baseline in same units
as numerator (time or volume)
Resolution
Resolution
VN (a - 1)
N= number of plates
a = relative retention
k = retention factor for second peak
Resolution =
(1 + k2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning