Determine the enthalpy for this reaction: Express your answer in kilojoules per mole to one decimal place. Ca(OH)2 (s) + CO2(g) →CaCO3 (s) + H₂O(1)
Determine the enthalpy for this reaction: Express your answer in kilojoules per mole to one decimal place. Ca(OH)2 (s) + CO2(g) →CaCO3 (s) + H₂O(1)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 44E: Although the gas used in an oxyacetylene torch (Figure 5.7) is essentially pure acetylene, the heat...
Related questions
Question
100%
Pls help ASAP. Pls give proper units and significant digits to three. Pls circle the final answer as well.
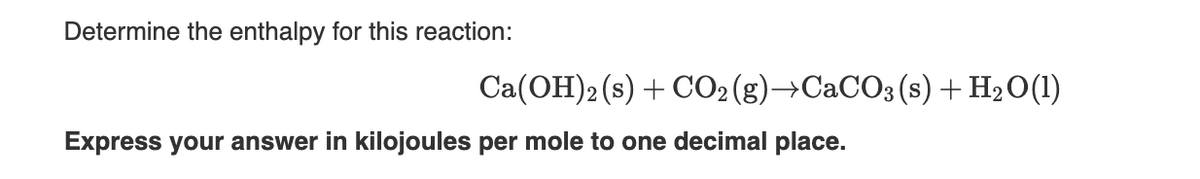
Transcribed Image Text:Determine the enthalpy for this reaction:
Express your answer in kilojoules per mole to one decimal place.
Ca(OH)2 (s) + CO2(g) →CaCO3 (s) + H₂O(1)
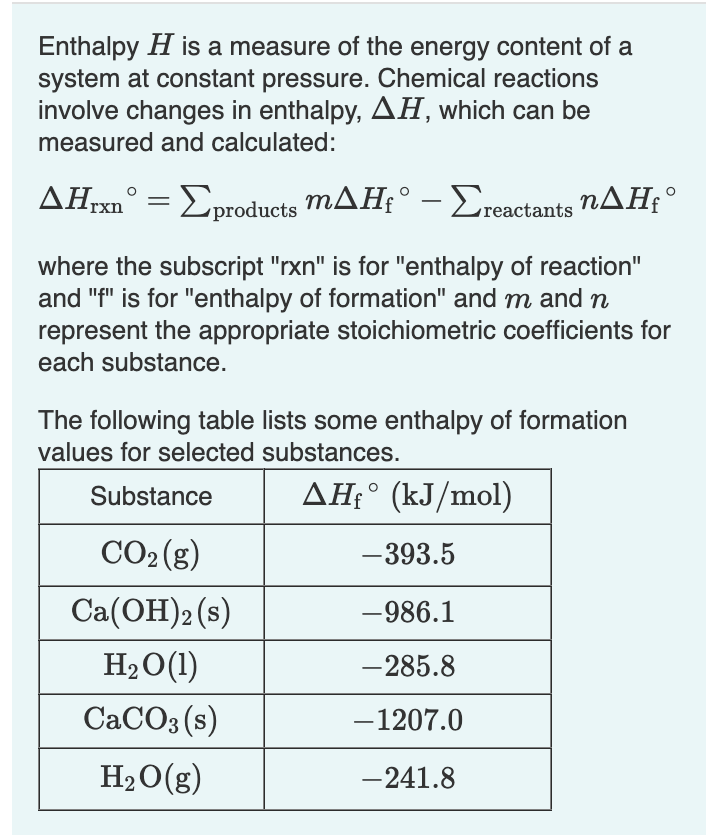
Transcribed Image Text:Enthalpy H is a measure of the energy content of a
system at constant pressure. Chemical reactions
involve changes in enthalpy, AH, which can be
measured and calculated:
ΔΗ,xn° = Σproducts mΔΗ;° - Σreactants nΔΗ,°
where the subscript "rxn" is for "enthalpy of reaction"
and "f" is for "enthalpy of formation" and m and n
represent the appropriate stoichiometric coefficients for
each substance.
The following table lists some enthalpy of formation
values for selected substances.
Substance
AHfᵒ (kJ/mol)
CO₂(g)
-393.5
Ca(OH)2 (s)
-986.1
H₂O(1)
-285.8
CaCO3(s)
-1207.0
H₂O(g)
-241.8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,