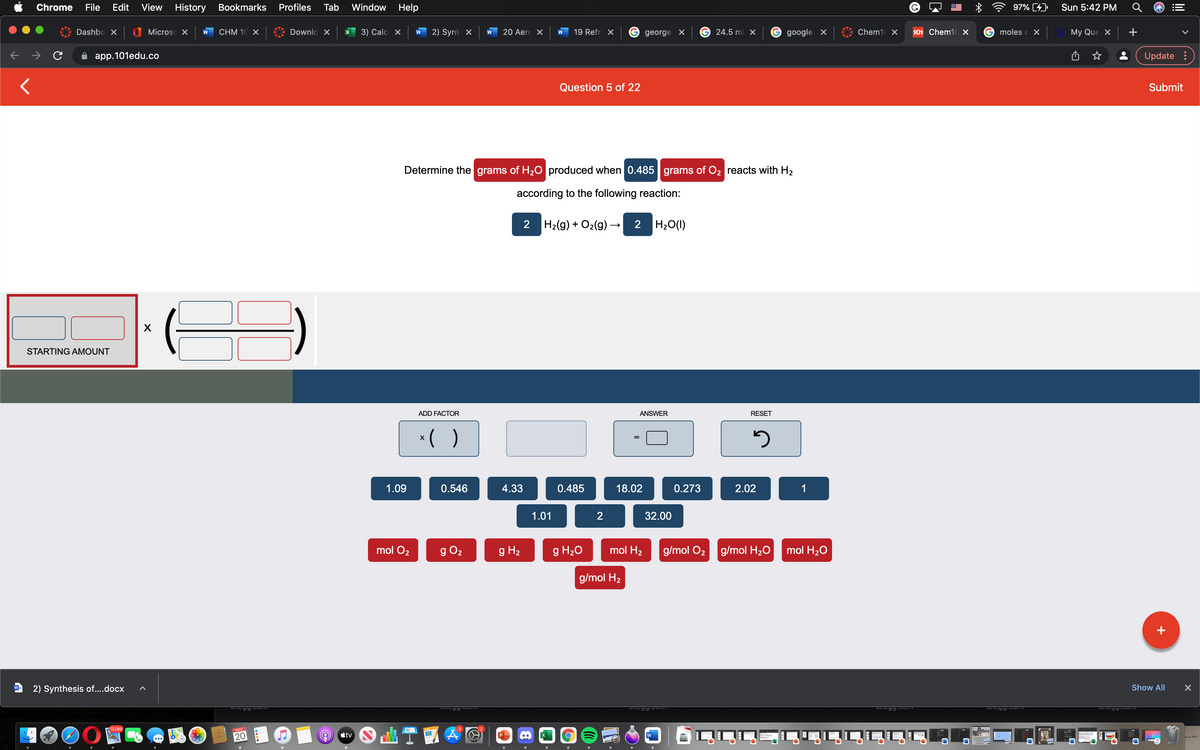
Determine the grams of H;0 produced when 0.485 grams of O; reacts with H2 according to the following reaction: 2 Ha(9) + O2(9) - 2 H,0(1) ADD FACTOR ANSWER RESET :( ) 1.09 0.546 4.33 0.485 18.02 0.273 2.02 1.01 32.00 9 H, 9 H,0 mol H; g/mol O; 9g/mol H;0 mol H,0 mol O, g/mol H2
Determine the grams of H;0 produced when 0.485 grams of O; reacts with H2 according to the following reaction: 2 Ha(9) + O2(9) - 2 H,0(1) ADD FACTOR ANSWER RESET :( ) 1.09 0.546 4.33 0.485 18.02 0.273 2.02 1.01 32.00 9 H, 9 H,0 mol H; g/mol O; 9g/mol H;0 mol H,0 mol O, g/mol H2
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 49A
Related questions
Question
100%
i need help with this
Transcribed Image Text:Chrome
File
Edit
View
History Bookmarks
Profiles
Tab
Window Help
97% [4)
Sun 5:42 PM
Dashbo X
Microsc X
CHM 10Χ
Downlo X
3) Calci X
2) Synt X
20 Aerc X
19 Refri X
24.5 ml X
google X
Chem1 X
101 Chem1 X
moles c X
My Que X
+
W
george X
app.101edu.coо
Update :
Question 5 of 22
Submit
Determine the grams of H20 produced when 0.485 grams of O2 reacts with H2
according to the following reaction:
2 H2(g) + O2(g) –
2 H20(1)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
1.09
0.546
4.33
0.485
18.02
0.273
2.02
1
1.01
2
32.00
mol O2
g O2
g H2
g H20
mol H2
g/mol O2 g/mol H20
mol H20
g/mol H2
+
2) Synthesis of....docx
Show All
T國
13,088
MAR
20
étv
P
w
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning