Determine the mass in grams of C«H10 that are required to completely react to produce 8.70 mol of CO: according to the following combustion reaction: 2 C-H:0(g) + 13 0:(g) 8 CO:(g) + 10 H:O(g)
Determine the mass in grams of C«H10 that are required to completely react to produce 8.70 mol of CO: according to the following combustion reaction: 2 C-H:0(g) + 13 0:(g) 8 CO:(g) + 10 H:O(g)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.44PAE: 4.44 Industrial production of hydrogen gas uses the reaction shown below. If 1.00 metric ton of...
Related questions
Question
Transcribed Image Text:O How to find the Number of Ator X
+
G Determine the formula weight of X
S How many grams are there in 1. X
gle Search
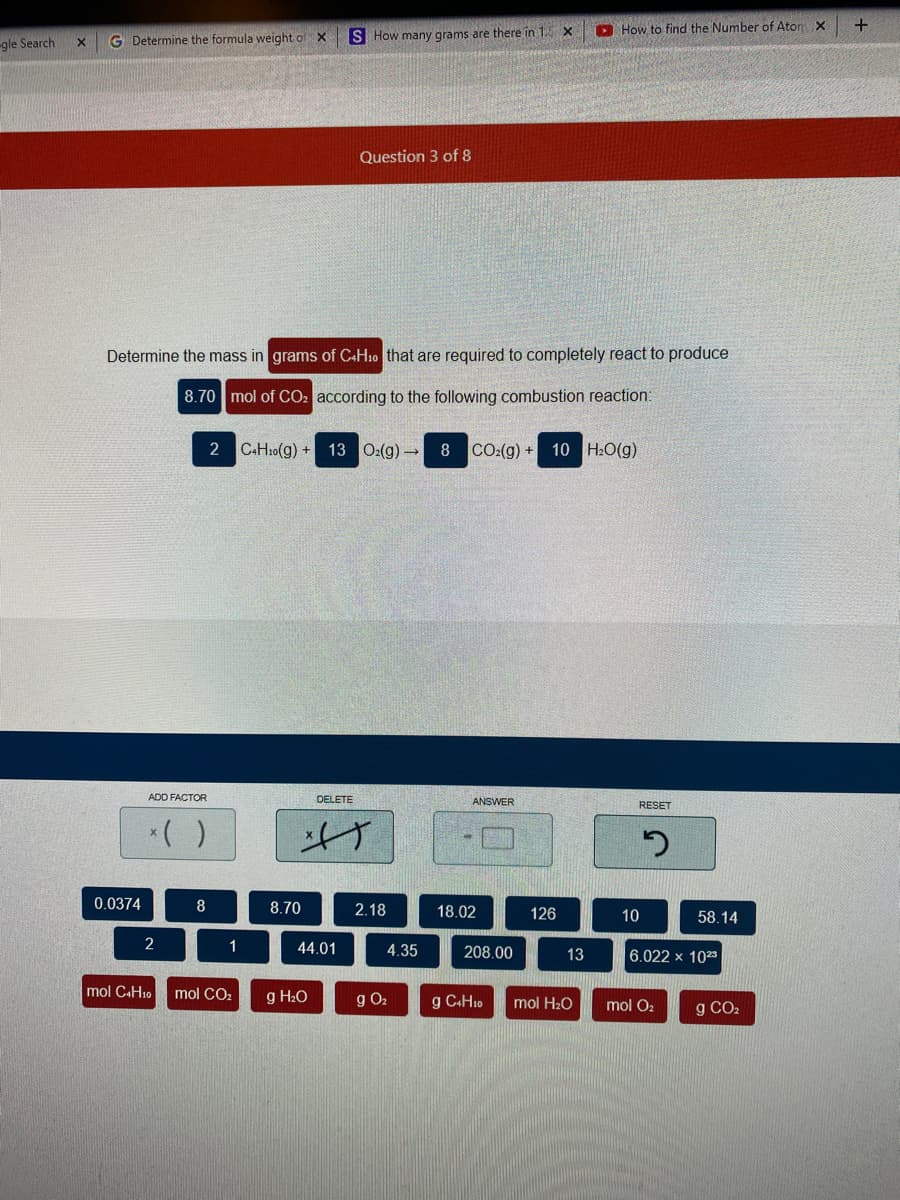
Question 3 of 8
Determine the mass in grams of C+H10 that are required to completely react to produce
8.70 mol of CO: according to the following combustion reaction:
2 C.H10(g) + 13 0:(g)→
8
CO:(g) + 10 H:O(g)
ADD FACTOR
DELETE
ANSWER
RESET
*( )
げ
0.0374
8
8.70
2.18
18.02
126
10
58.14
1
44.01
4.35
208.00
6.022 x 1023
13
mol CAH10
mol CO2
g H2O
g O2
g C+H10
mol H20
mol O2
g CO2
Expert Solution
Step 1
The balanced reaction given is,
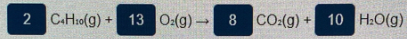
Given: Moles of CO2 produced = 8.70 mol.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning