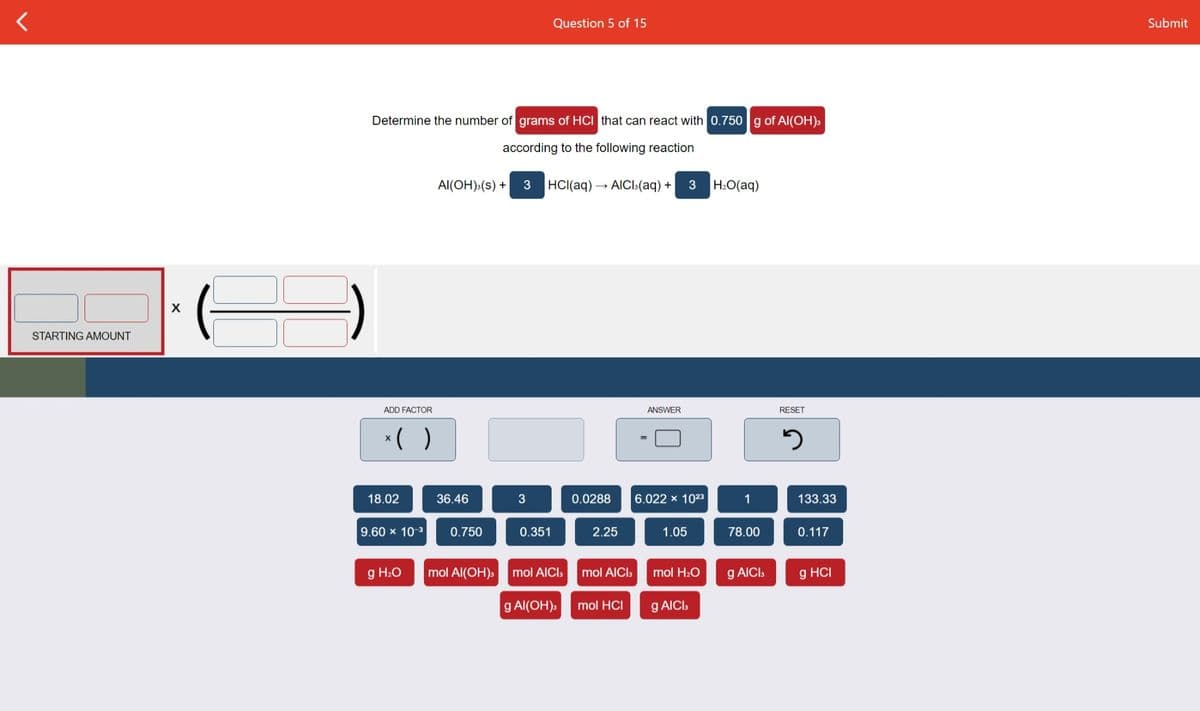
Determine the number of grams of HCI that can react with 0.750g of A(OH)» according to the following reaction Al(OH) (s) + 3 HCI(aq) - AICI(aq) + 3 H.O(aq)
Determine the number of grams of HCI that can react with 0.750g of A(OH)» according to the following reaction Al(OH) (s) + 3 HCI(aq) - AICI(aq) + 3 H.O(aq)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 65E: Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is...
Related questions
Question
Transcribed Image Text:Question 5 of 15
Submit
Determine the number of grams of HCI that can react with 0.750 g of Al(OH):
according to the following reaction
Al(OH):(s) +
3
HCI(aq) → AICI3(aq) +
H2O(aq)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
18.02
36.46
0.0288
6.022 x 1023
1
133.33
9.60 x 10-3
0.750
0.351
2.25
1.05
78.00
0.117
g H2O
mol Al(OH)s
mol AICI.
mol AICIs
mol H2O
g AICIS
g HCI
g Al(OH),
mol HCI
g AICI,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning