Q: Provide an equilibrium constant for this reaction. OLi H. Li-N H-N pKa - 23 pka - 36 A. 1059 В.…
A: This is an acid base reaction where HA is the acid and B is the base. A- is the conjugate base and…
Q: Calculate the pH of a buffer solution containing 10.0 cm3 of 0.100 mol dm–3 NaOH and 20.0 cm3 of…
A:
Q: Which information is not found in an MSDS/SDS? * Buyer Information Accidental Release Measures…
A:
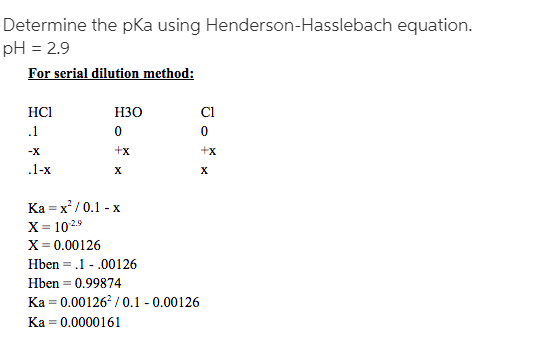
Q: How was the theoretical value of the pKa determined? In that given graph
A: Explanation is given in the following step.
Q: Proteins are characterized by their isoelectric point (pl), at which they carry no net charge.…
A: Protein A has pI = 5.1
Q: A monoprotic weak acid, HA, dissociates in water according to the reaction HA(aq) = H*(aq) + A¯(aq)…
A: Given, the dissociation equation for the weak acid, HA: HA (aq) ⇌ H+ (aq) + A- (aq) Given the…
Q: The K, for acetic acid (CH,COOH) is 1.737 x 10-. What is the pK for this acid? pKa = Use the…
A: 1) Ka(CH3COOH) = 1.737 x 10-5 pKa = -logKa = -log(1.737 x 10-5) pKa =…
Q: Which one of the following indicators is best suited for titrating 0.40 M ammonia (pKb = 4.74) with…
A: The chemical reaction occurs in the given titration (strong acid+weak base) is: NH3 + HCl = NH4Cl…
Q: A general expression for Alpha values Consider a 1.64 M solution of Phtalic acid C6H4 (CO2H)2…
A: Hello. Since your question has multiple sub-parts, we will solve the first three sub-parts for you.…
Q: In what buffer system (PAH, SAD, or DOH) will Phoebe store the enzyme? (show calculations and…
A: The pH of a buffer solution can be determined by Henderson's equation given below. pH=pKa +…
Q: Why did pCO2 decrease in the tank with added CO32-
A: Le Chatelier's principle is used to predict the effect of a change in conditions on chemical…
Q: Four tablet samples (1.6308 g) which contain acetylsalicylic acid were dissolved in a 100 mL…
A:
Q: What is the pH of a solution containing 0.642 mol L-1 of a weak acid with pKA = 3.01 and 0.865 mol…
A:
Q: Room Temperature: 21°C Pressure: 756.6660 mmHg Note: In this experiment, the standardized acetic…
A:
Q: A mixture of 0.15 M acetic acid and 0.34 sodium acetate is given. Calculate the pH of the medium if…
A: The given data contains, Acetic acid = 0.15 M. Sodium acetate = 0.34 M. pKa of acetic acid = 4.87
Q: Show how the composition of an aqueous solution that contains 20 mmol dm-3 glycine varies with pH .…
A: Given data: [H3N-CH2-COOH] = 20 mmol dm-3 pKa1 = 9.60 pKa2 = 2.35 Calculation of Ka1 and Ka2: pKa1 =…
Q: Reaction: 2HCI + Ca(OH)2 →2H20 + CaCl2 Initial Burette Volume of 0.O5M HCI Volume of saturated…
A: Molarity is defined as total number of solute present in 1000 ml of solution . For neutralisation we…
Q: A lake water is analyzed and found to have 1.2 mg NH4*/L, at pH = 7.8. a. Using pKa and ionization…
A: pKa for NH4+ ions is equal to 9.26.
Q: 5.00 mL of a water sample was titrated for hardness with 4.15 mL of 0.0200 M EDTA. How many moles…
A: Given data,Molarity of EDTA=0.02MVolume of EDTA=4.15mL=0.00415L
Q: 2. Phoebe was tasked to ensure that an enzyme responsible for keeping her species from extinction…
A:
Q: what is the liquid-liquid extraction efficiency of Amphetamin (pka= 9.8) taken from urine into…
A:
Q: The Ksp of BaSO4 is 1.7 x 10-10. Calculate the concentration of the Ba+2 and SO4-2.
A: Since you have posted multiple questions, we will solve first one for you. To get remaining…
Q: A 31.6 mL aliquot of hypochlorous acid that has a concentration of 0.204 M will be titrated with…
A: To calculate pH, we would first Calculate moles of both acid and base . Then we would draw an ICE…
Q: If the base (NaOH) is standardized to 0.12 M in Part A of this experiment, calculate the amount of…
A: The reaction between oxalic acid dihydrate and NaOH is ; H2C2O4·2H2O + 2NaOH → Na2C2O4 + 4H2O From…
Q: 5. A mixture of 0.20 Macetic acid and 0.30 Msodium acetate is given. What the pH of the medium if…
A:
Q: Which conjugate acid/base pair could be used to create a pH 3.20 buffer solution with the largest…
A: Buffer works well when - pH = pKa Ratio of [Conjugate base] / [Acid] = 1:1
Q: The Ksp value for magnesium hydroxide is 5.61 × 10–12 at 25 °C. In a saturated solution of magnesium…
A:
Q: The K, for acetic acid (CH,COOH) is 1.737 × 10-5. What is the pK, for this acid? pKa = Use the…
A: We know that, pKa is the negative logarithm of Ka. i.e. pKa= -logKa We have, Ka…
Q: 1) Using the measured pH, calculate the concentration of H3O+ and OH- at the equivalence point.
A: As per regulations we are only supposed to answer only one question.
Q: A monoprotic weak acid, HA, dissociates in water according to the reaction HA(aq) = H*(aq) + A (aq)…
A:
Q: Injected a patient with isotopic water concentration 100mg, after 2 hours of equilibrium the…
A: Amount of isotopic water given to the patient = 100 mg Amount of isotopic water excreted or lost in…
Q: 2. Phoebe was tasked to ensure that an enzyme responsible for keeping her species from extinction…
A: Buffer solution is mixture of weak acid and salt of weak acid or weak base and salt of weak acid. It…
Q: Which solution is closest to the pKa? Calculate the pH of the solutions and the change in pH after…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: *D. Ksp = [Ag*]² · [SO4 1 %3D Ksp- pwidut/deacte.cauh una bulilor liguel. 2. If the gram solubility…
A:
Q: Find the pKa.pKa. For an acid HA, the concentrations of HA and A- A are 0.075 and 0.025,…
A: Given Values: Concentration of HA = 0.075 M Concentration of A- = 0.025 M pH of the buffer = 6.0
Q: A student determines that the value of Ka for H2SO3 = 2.3×10-2 . What is the value of pKa?
A: pKa is the measure of determining the strength of the acid. Lower the value of pKa, stronger will be…
Q: Which type of indicator is used in bromatometry: irreversible Red/Ox indicator; adsorption…
A: Option 3 is the correct Choice. Bromatometry falls in the class of redox titration.
Q: pH = 7.15 + log [0.25]/[0.50] pH = pKa + log [0.33]/[0.11]; (Ka = 6.3096 x 10-8) [OH-] = 2.78 x…
A: Given : a. pH = 7.15 + log [0.25]/[0.50] b. pH = pKa + log [0.33]/[0.11]; (Ka = 6.3096 x 10-8) c.…
Q: 2. Phoebe was tasked to ensure that an enzyme responsible for keeping her species from extinction…
A: Since you have posted a question with multiple sub-parts, we will solve the first three sub-parts…
Q: SOLUBILITY PRODUCT PRINCIPLE Calcium phosphate (Ca3(PO4)2; MM = 310.18 g/mol), a large component of…
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: pKa
A:
Q: Use the method of successive approximations to determine the pH and concentrations of H₂A, HA, and…
A:
Q: You are testing the efficacy of different treatments on eliminating SARS-Cov-2 from surfaces. Which…
A: While testing the efficacy of different treatments on eliminating SARS-Cov-2 from surfaces. the…
Q: 22 g of potato salad was added to 60 mLs of buffer and blended. Three (1/10) dilutions of the…
A:
Q: The theoretical pka of a weak acid is 3.54. Calculate the pKa experimental error of a solution…
A:
Q: you'd like to weigh out acetic acid and sodium acetate (pka=4.75) to use as buffer system to study…
A: This would simply require the Henderson Hasslebech reaction, which states that when the pKa value of…
Q: Determination of Ksp of CaCO3 based on the measured pH and the following equations: CaCO3 (s) Ca 2+…
A: Here in this question some informations are given (Concentration value of Ca2+and PH value of the…
Q: The dissolution of borax is: Na2B4O5(OH)4 • 8H2O(s) ⇌ 2 Na+(aq) + B4O5(OH)42–(aq) + 8 H2O(l)…
A: According to the mole concept, in terms of mass, the amount of substance in moles is equal to the…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Q) My water hardness experiment is wrong? I did hard water, tap water, and soft water experiment. I mixed hard / soft / tap water 5ml + pH buffer 1ml + 3 drops of indicator + EDTA x ml My expectation was, Hard water will need most EDTA, then tap water, then soft water. However, my experiment result was EDTA of hard water : 0.15ml EDTA of tap water : 0.33ml EDTA of soft water : 0.03ml Also, when I mixed soft water 5ml + + pH buffer 1ml + 3 drops of indicator ; without EDTA, it was already purple color. I tried this for 4 time to make color pink, but I couldn't. The number 0.03ml is ml to change 'purple to blue'. Q1) My result of each water's EDTA ml is reasonable? Q2) soft water without EDTA color can be purple? Thank you.Ali has measured the oxidative stability of palm oil sample under accelerated storage condition at 60*C for 6 days The following data were recorded for the peroxide value and p anisidine value of the palm oil after 5 days of storage time: Sample weight 1.0030 g Concentration of NazSzO3 = 0.001 N Volume of NazSzO3 titrated for sample solution = 1.35 ml Volume of NazSzO3 titrated for blank solution 0.20 ml Absorbance for sample solution = 0.907 Absorbance for blank solution 0.203 Calculate: i) peroxide value ii) p-anisidine value iii) TOTOX valueCalculate the [FeSCN2+] using volumes of stock solutions. Presume that all the SCN– ions react. STANDARD SAMPLE Volume of Fe(NO3)3 (mL) Volume of SCN- (mL) Volume of H2O (mL) [FeSCN2+] Absorbance 1 2.5 2.0 20.5 0.1918 2 2.5 1.5 21.0 0.3239 3 2.5 1.0 21.5 0.4965 4 2.5 0.5 22.0 0.6209 Stock [Fe(NO3)3] = 0.200 M, Stock [SCN-] = 0.0020 M Any help would be greatly appreciated Please and thanks :)
- A 100 ml bottle of metronidazole 100 mg/ml suspension is available in your pharmacy. Calculate the volume of this suspension needed to be diluted with cherry syrup to prepare 60 ml of a 4% metronidazole suspension. 1 ml of a 1:1000 epinephrine injection was mixed with 20 ml of 1% lidocaine injection. Calculate the new ratio strength of epinephrine in the admixture. (IGNORE ANY VOLUME CHANGES AFTER ADMIXTURE) Instead of preparing 4 grams of tetracaine hydrochloride 4% gel an 8% gel was compounded by mistake. How many grams of the 8% gel and gel base must be mixed to get 4 grams of a 4% gel? How many milliliters of 70% ethanol and 20% ethanol must be mixed to prepare 500 ml of 30% ethanol? A diphenhydramine elixir contains 12.5 mg drug in one teaspoon. The volume of oral vehicle that is to be added to 100 ml of this elixir to reduce its strength by one half its original strength is; 6. 90 capsules of Liothyronine (T3) 15 micrograms are to be prepared. The formula calls for a 1:10000…Calculate the mass in grams of Al (FM = 26.98) present in an unknown sample if 35.79 mL of 0.8765 M EDTA was required to reach the Calmagite endpoint. Group of answer choices 0.6607 g 0.8464 g 1.163 g 1.102 gExtraction of DNA involves the use of a 1 M phosphate buffer (1-liter volume) having the hydrogen ion concentration of 6.165950019 x 10-9 M. Three pKa of phosphoric acid are available Concentrated phosphoric acid (85% w/w; Sp. Gr. = 1.70; MW = 98 g/mol) Sodium dihydrogen phosphate monohydrate (MW = 138g/mol) Sodium biphosphate heptahydrate (MW = 268g/mol) Tribasic sodium phosphate monohydrate (MW = 182 g/mol) Sodium hydroxide pellets (40 grams/mol) Question: What is the pH of the solution? (answer in two decimal places) How much of the weak acid component should be used? (Note for answer: Round to the nearest hundredth and include the unit such as "grams or milliliters"; Ex. 100.00 grams or 100.00g for weight & 100.00 milliliters or 100.00 mL for volume) Question: What is the suitable buffer component to be used? (Note for the answer: Separate the two components with “and”; Ex: Ammonia and Ammonium chloride) Question: How much of the salt component should be used?…
- The potentiometric titration data of 2,422 mmol chloride ion with 0.1000 M AgNO3 are as follows. What should the x and y axis values be to find the turning point using the first derivative curve? AgNO3 volume (mL) Potential (Volts) 5.0 0.062 15.0 0.085 20.0 0.107 22.0 0.123 23.0 0.138 23.50 0.146 23.80 0.161 24.00 0.174 24.10 0.183 24.20 0.194 24.30 0.233A solution of Ba(OH)2 was standardized against0.1215 g of primary-standard-grade benzoic acid, C6H5COOH (122.12 g/mol). An end point was observedafter addition of 43.25 mL of base. (a) Calculate the molar concentration of the base. (b) Calculate the standard deviation of the molarconcentration if the standard deviation for themass measurement was ±0.3 mg and that for thevolume measurement was ±0.02 mL. (c) Assuming an error of ±0.3 mg in the mass measurement,calculate the absolute and relative systematicerror in the molar concentration.Nick and Kel reacted 2.80g of NiCl2 x 6H20 with 7.00 ml of 4.00 M C2H8N2. They recovered 2.40g of product, [Ni(C2H8N2)3] Cl2. At the end, the filtrate was a dark blue solution. The litmus test on the filtrate solution left the red litmus paper unchanged in color. - NiCl2 x 6H2O + 3C2H8N2 yields [Ni(C2H8N2)3] Cl2(s) + 6H20 - Molor mass of NiCl2 x 6H20 is 237.69 g/mol - Molarity of C2H8N2 is 4.00 M - Molor mass of product,[Ni(C2H8N2)3] Cl2(s) is 309.90 g/mol based on the experiment described, what are answers for the question below the actual or experimenting yield is ________ the theoretical yeild is _____
- Nick and Kel reacted 2.80g of NiCl2 x 6H20 with 7.00 ml of 4.00 M C2H8N2. They recovered 2.40g of product, [Ni(C2H8N2)3] Cl2. At the end, the filtrate was a dark blue solution. The litmus test on the filtrate solution left the red litmus paper unchanged in color. - NiCl2 x 6H2O + 3C2H8N2 yields [Ni(C2H8N2)3] Cl2(s) + 6H20 - Molor mass of NiCl2 x 6H20 is 237.69 g/mol - Molarity of C2H8N2 is 4.00 M - Molor mass of product,[Ni(C2H8N2)3] Cl2(s) is 309.90 g/mol based on the experiment described, what are answers for the question below the actual or experimenting yield is __2.40g______ the theoretical yeild is __2.89g___ (can someone show me how to caluculate to get this answer?)4. determine the amount in mg of the amount of Ag present from ICP mass digested: 1.0821g/L dilution of 10ml stock solution to 100ml volumetric flask X=0.98 (intensity)You obtained the following raw data when setting up a Bradford standard curve: BSA (mg/ml) Absorbancy 595nm 0 0.225 1 0.310 2 0.420 3 0.510 4 0.610 5 0.720 6 0.810 7 0.915 8 0.950 9 0.980 10 0.990 After blanking against a bradford-dH2O sample, the protein concentration of an unknown sample was determined using the same method and an absorbancy of 0.523 was obtained. Set up a standard curve, excluding outliers (experimental and statistical) and determine the protein concentration in the unknown sample in mg / ml (up to 3 significant figures).





