Diamond is one of the many allotropes of carbon. At high temperatures, diamond can be oxidized by oxygen in the atmosphere. Write the balanced chemical equation for this combustion reaction.
Diamond is one of the many allotropes of carbon. At high temperatures, diamond can be oxidized by oxygen in the atmosphere. Write the balanced chemical equation for this combustion reaction.
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 9.9EP: Indicate to which of the following types of reactions each of the statements listed applies:...
Related questions
Question
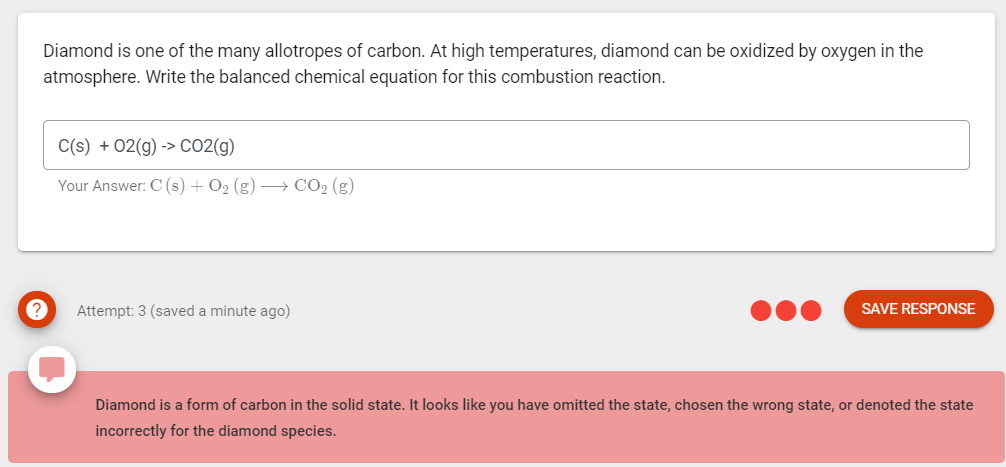
Transcribed Image Text:Diamond is one of the many allotropes of carbon. At high temperatures, diamond can be oxidized by oxygen in the
atmosphere. Write the balanced chemical equation for this combustion reaction.
C(s) + 02(g) -> CO2(g)
Your Answer: C (s) + O2 (g) –→ CO2 (g)
Attempt: 3 (saved a minute ago)
SAVE RESPONSE
Diamond is a form of carbon in the solid state. It looks like you have omitted the state, chosen the wrong state, or denoted the state
incorrectly for the diamond species.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning