diprotic acid. The procedure for identifying an unknown acid is exactly as described in the lab manual. Part 1: Standardization of NaOH. 1.4378 g of oxalic acid was dissolved in 250.0 mL of water and a 25.00 m aliquote was titrated with NaOH. 24.73 mL of titrant was required. Determine the concentration of sodium hydroxide. Answer: Part 2: Titration of the unknown acid. 1.9875 g of the unknown acid was dissolved in 250.0 ml of water and 25.00 ml of this solution was titrated with the NaOH. 29.50 mL of titrant was required. Determine the Molar Mass of the unknown. Answer:
diprotic acid. The procedure for identifying an unknown acid is exactly as described in the lab manual. Part 1: Standardization of NaOH. 1.4378 g of oxalic acid was dissolved in 250.0 mL of water and a 25.00 m aliquote was titrated with NaOH. 24.73 mL of titrant was required. Determine the concentration of sodium hydroxide. Answer: Part 2: Titration of the unknown acid. 1.9875 g of the unknown acid was dissolved in 250.0 ml of water and 25.00 ml of this solution was titrated with the NaOH. 29.50 mL of titrant was required. Determine the Molar Mass of the unknown. Answer:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 77QAP: Two students were asked to determine the Kb of an unknown base. They were given a bottle with a...
Related questions
Question
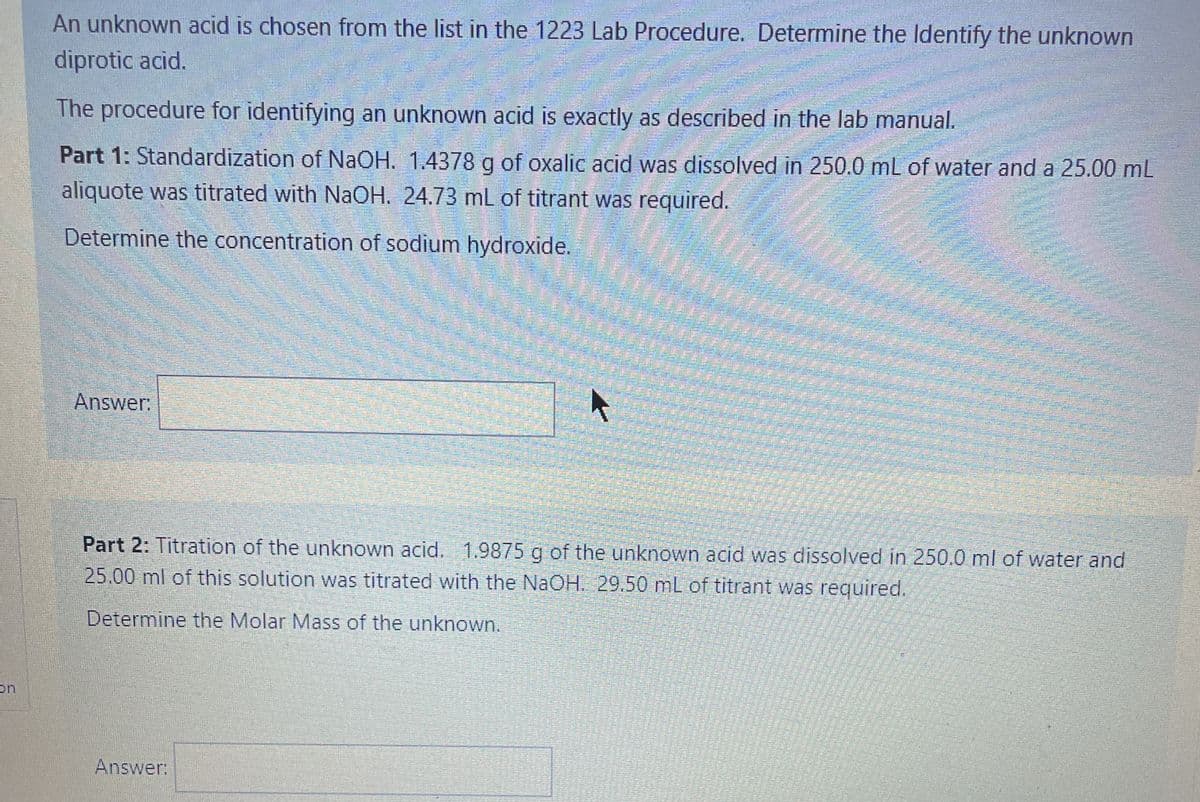
Transcribed Image Text:An unknown acid is chosen from the list in the 1223 Lab Procedure. Determine the Identify the unknown
diprotic acid.
The procedure for identifying an unknown acid is exactly as described in the lab manual.
Part 1: Standardization of NaOH. 1.4378 g of oxalic acid was dissolved in 250.0 mL of water and a 25.00 mL
aliquote was titrated with NaOH. 24.73 mL of titrant was required.
Determine the concentration of sodium hydroxide.
Answer:
Part 2: Titration of the unknown acid. 1.9875 g of the unknown acid was dissolved in 250.0 ml of water and
25.00 ml of this solution was titrated with the NaOH. 29.50 mL of titrant was required.
Determine the Molar Mass of the unknown.
on
Answer:
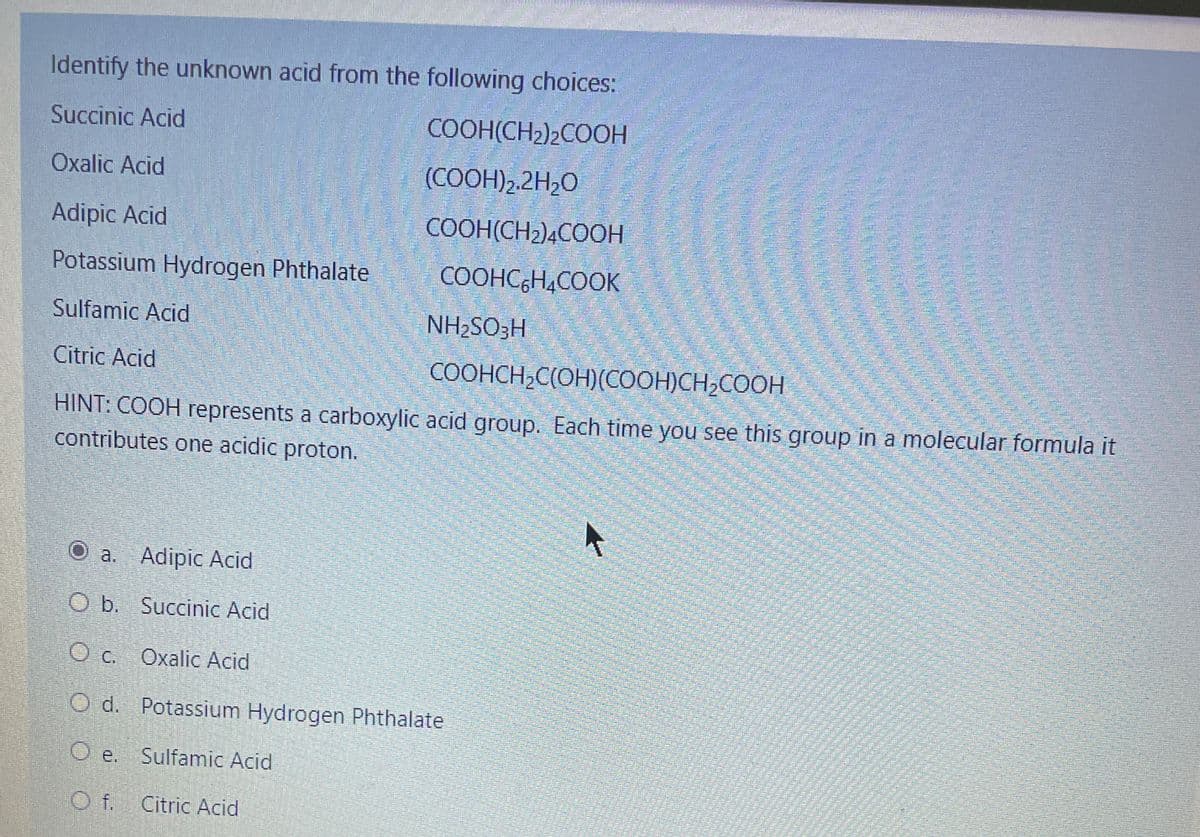
Transcribed Image Text:Identify the unknown acid from the following choices:
Succinic Acid
COOH(CH2)2COOH
Oxalic Acid
(COOH)2.2H,O
Adipic Acid
COOH(CH2)4COOH
Potassium Hydrogen Phthalate
COOHC,H,COOK
Sulfamic Acid
NH2SO;H
Citric Acid
COOHCH,C(OH)(COOH)CH;COOH
HINT: COOH represents a carboxylic acid group. Each time you see this qgroup in a molecular formula it
contributes one acidic proton.
O a. Adipic Acid
O b. Succinic Acid
O c. Oxalic Acid
O d. Potassium Hydrogen Phthalate
O e. Sulfamic Acid
O f. Citric Acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning