Directions: Classify the species into three categories: ionic, polar (possessing a dipole moment), and nonpolar and identify what type(s) of intermolecular forces exist between the following pairs, molecules (or basic units). (Keep in mind that dispersion forces exist between all species.)
Directions: Classify the species into three categories: ionic, polar (possessing a dipole moment), and nonpolar and identify what type(s) of intermolecular forces exist between the following pairs, molecules (or basic units). (Keep in mind that dispersion forces exist between all species.)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.92PAE
Related questions
Question
ANSWER 1,2&3 ONLY.
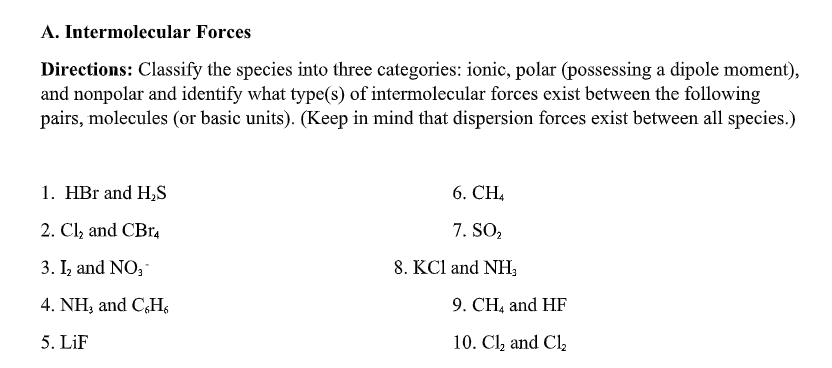
Transcribed Image Text:A. Intermolecular Forces
Directions: Classify the species into three categories: ionic, polar (possessing a dipole moment),
and nonpolar and identify what type(s) of intermolecular forces exist between the following
pairs, molecules (or basic units). (Keep in mind that dispersion forces exist between all species.)
1. HBr and H,S
6. CH,
2. Cl, and CBr,
7. SO2
3. I, and NO,
8. KCl and NH,
4. NH, and CHs
9. CH, and HF
5. LiF
10. Cl, and Cl,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning