Dissolution 0.01 M HCI Distilled H20 Concentration (mg/ml) Total amount Concentration (mg/ml) Time A Total amount A (mg) (mg) 0.16 0.1 10 0.24 0.18 15 0.35 0.25 20 0.48 0.34 0.4 0.5 25 0.58 30 0.67
Dissolution 0.01 M HCI Distilled H20 Concentration (mg/ml) Total amount Concentration (mg/ml) Time A Total amount A (mg) (mg) 0.16 0.1 10 0.24 0.18 15 0.35 0.25 20 0.48 0.34 0.4 0.5 25 0.58 30 0.67
Chapter6: The Systematic Approach To Equilibria: Solving Many Equations
Section: Chapter Questions
Problem 10P
Related questions
Question
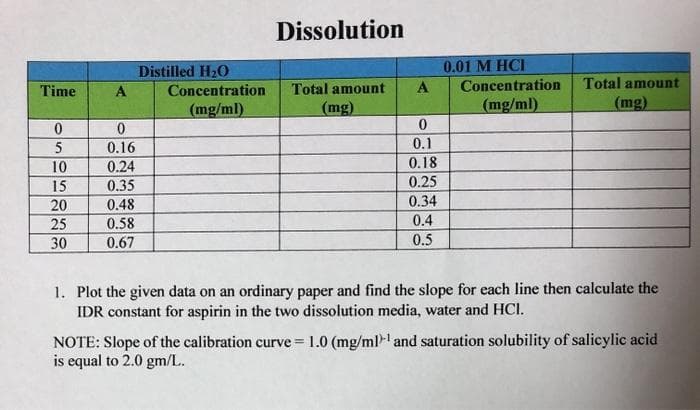
Transcribed Image Text:Dissolution
Distilled H20
Concentration
0.01 M HCI
Concentration
(mg/ml)
Time
Total amount
A
Total amount
A
(mg/ml)
(mg)
(mg)
5
0.16
0.1
10
0.24
0.18
15
0.35
0.25
20
0.48
0.34
25
0.58
0.4
30
0.67
0.5
1. Plot the given data on an ordinary paper and find the slope for each line then calculate the
IDR constant for aspirin in the two dissolution media, water and HCI.
NOTE: Slope of the calibration curve = 1.0 (mg/ml and saturation solubility of salicylic acid
is equal to 2.0 gm/L.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning