Do the exercises below on separate sheets of graph paper. Remember to use the entire sheet, to graph the values and use breaks where appropriate. 1. Use the following data to complete this graph regarding a gas. Volume (mL) Pressure (Torr) 33.3 600 25.0 800 20.0 1000 16.7 1200 14.3 1400 12.5 1600 11.1 1800 10.0 2000 2. Graph the solubility of KCIO2 at the various temperatures. Temperature (°C) Solubility (grams per 100 grams water) 1 20 40 17 60 27 80 39 100 57 3. Graph the vapor pressure of water using the following temperatures. Temperature (K) Vapor Pressure (Torr) 273 4.6 293 17.5 313 55.0 333 149.2 353 355.5 373 760.0
Do the exercises below on separate sheets of graph paper. Remember to use the entire sheet, to graph the values and use breaks where appropriate. 1. Use the following data to complete this graph regarding a gas. Volume (mL) Pressure (Torr) 33.3 600 25.0 800 20.0 1000 16.7 1200 14.3 1400 12.5 1600 11.1 1800 10.0 2000 2. Graph the solubility of KCIO2 at the various temperatures. Temperature (°C) Solubility (grams per 100 grams water) 1 20 40 17 60 27 80 39 100 57 3. Graph the vapor pressure of water using the following temperatures. Temperature (K) Vapor Pressure (Torr) 273 4.6 293 17.5 313 55.0 333 149.2 353 355.5 373 760.0
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 3CLE: Write a brief description of the relationships among each of the following groups of terms or...
Related questions
Question
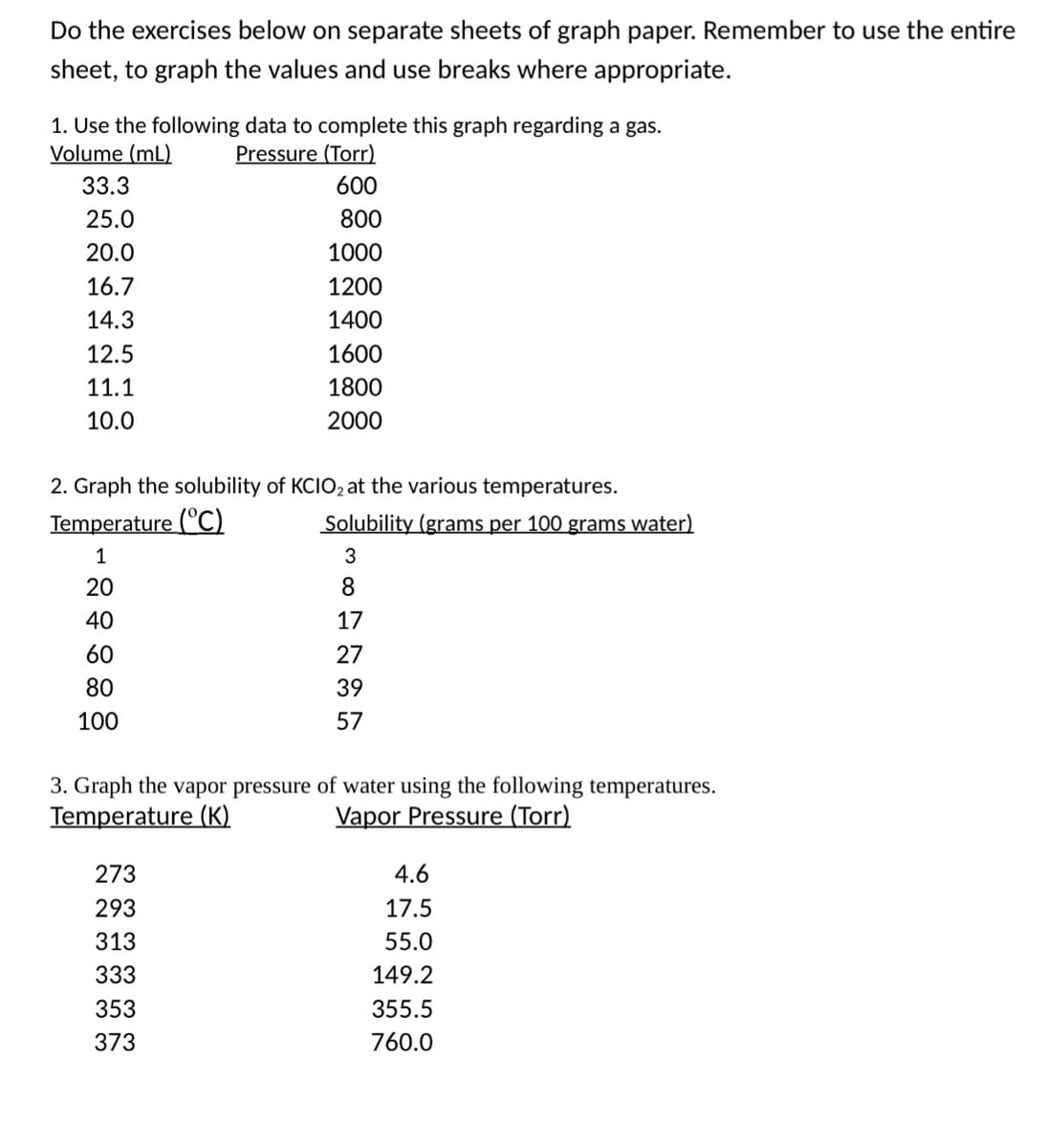
Transcribed Image Text:Do the exercises below on separate sheets of graph paper. Remember to use the entire
sheet, to graph the values and use breaks where appropriate.
1. Use the following data to complete this graph regarding a gas.
Volume (mL)
Pressure (Tor)
33.3
600
25.0
800
20.0
1000
16.7
1200
14.3
1400
12.5
1600
11.1
1800
10.0
2000
2. Graph the solubility of KCIO, at the various temperatures.
Temperature (C)
Solubility (grams per 100 grams water)
1
3
20
40
17
60
27
80
39
100
57
3. Graph the vapor pressure of water using the following temperatures.
Temperature (K)
Vapor Pressure (Torr)
273
4.6
293
17.5
313
55.0
333
149.2
353
355.5
373
760.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co