Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
100%
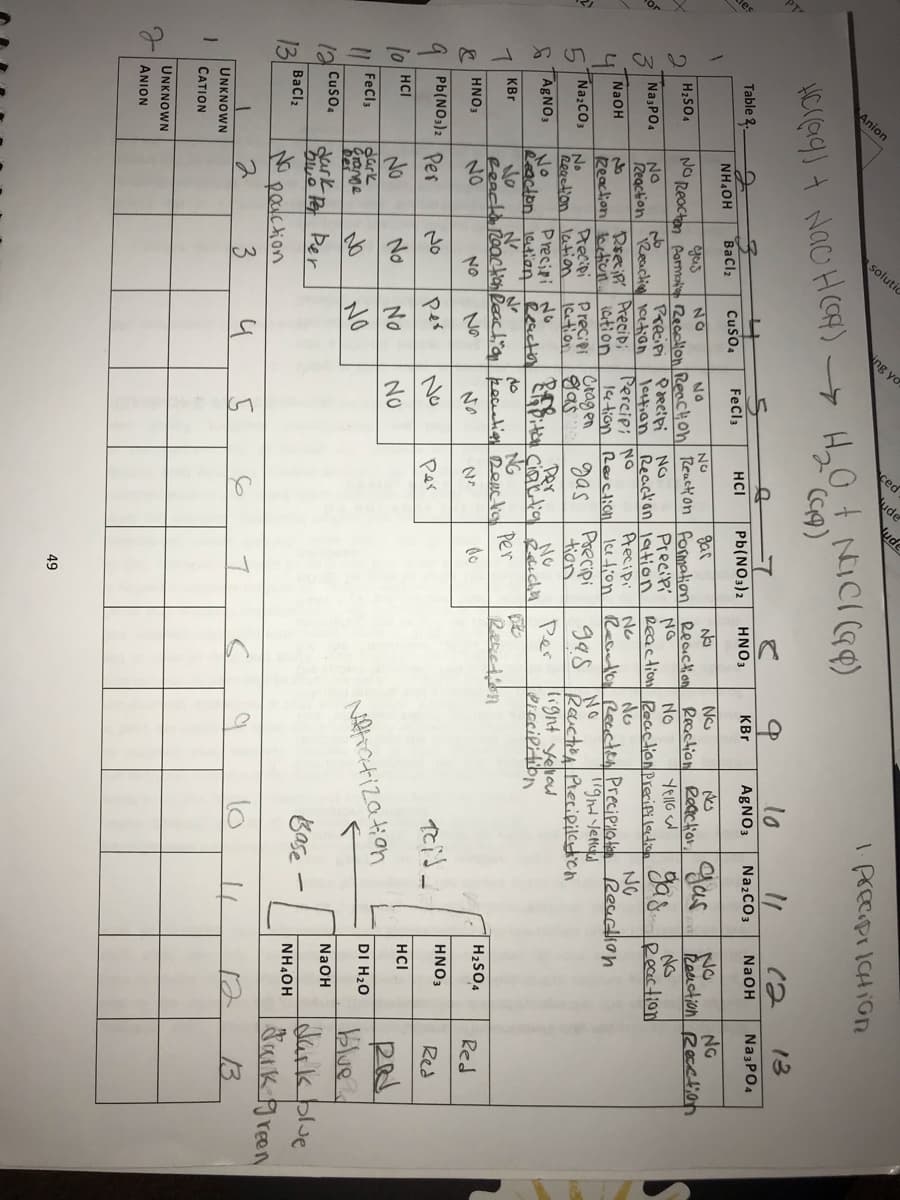
Do the solubility rules in your table agree with those given in the Ebbing and Gammon textbook? Note Similarities and differences .
Transcribed Image Text:Anion
solutic
ing yo
1.precipriCHiGn
PT
10
(2
13
es
Table 2.
Baclz
Cuso,
FeCla
HCI
Pb(NO3)2
HNO3
KBr
AGNO3
Na2CO3
NaOH
N 3PO4
NH.OH
gar
Reacton Porma Recellon Reachon Reaction fomation Reacion Recction Reactor aas
Precipi
NO
NO
NG
NO
Reuction Recctien
No
Reaction
NO
H2SO4
NO
No
NG
Reaction Recactionprecifilaticn
Precipi
Yello w
gag
Preeipi
|etion Reacton/ 19tion
Percip;
le tion Reaction le tion Recerfey Reacten Precipilat Recuction
Na PO4
NO
NO
Reaction Reachinn 1tion
Rrecip Precip;
Reaction oetiun iation
Precipi
Precipi
Ne
NG
NaOH
Na2CO3
Precipi Ctag en
Precipi
tien
gas
NO
No
Reaetion lation
No
gas Ractiea Precipilatich
gas
Ktion
Precipi No
Peacton latNan Reacto
E TAGNO,
Per
it ciactio
lignt velou
Piccipitibn
No
Per
KBr
do
Reache Roacien Reachion heuctigl Denctio Per
NO
HNO3
NO
Na
No
H2SO4
Red
9 Pb(NOs)2 Pes
Per
No
Per
HNO3
Red
HCI
No
dark
No
No
NO
HCI
FeCl3
NO
Nftiatization
DI H20
Blue
Sark blue
Rarkgreen
Cuso,
dark
NaOH
Baclz
NO
Base :
paction
NHẠOH
3
UNKNOWN
13
CATION
UNKNOWN
ANION
49
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning