ASAP: Please help me solve for Average Molarity of EDTA Standard Solution and Ca Titer (mg Ca/mL of EDTA Solution). Show step by step solution. This is the data.
ASAP: Please help me solve for Average Molarity of EDTA Standard Solution and Ca Titer (mg Ca/mL of EDTA Solution). Show step by step solution. This is the data.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter14: Mixtures And Solutions
Section14.3: Factors Affecting Solvation
Problem 42SSC
Related questions
Question
ASAP:
Please help me solve for Average Molarity of EDTA Standard Solution and Ca Titer (mg Ca/mL of EDTA Solution). Show step by step solution.
This is the data.
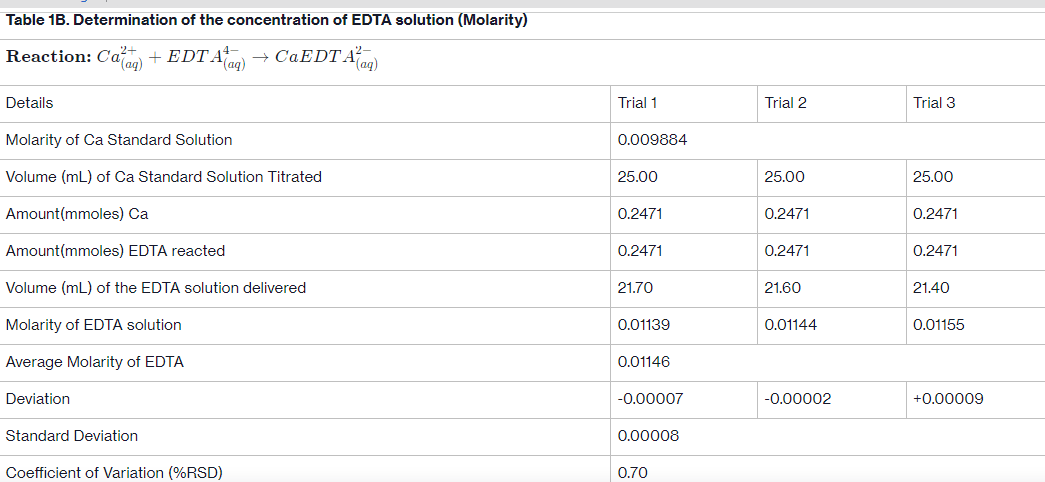
Transcribed Image Text:Table 1B. Determination of the concentration of EDTA solution (Molarity)
Reaction: Ca(aq) + · EDT Alaq) → CaEDTA²(aq)
Details
Molarity of Ca Standard Solution
Volume (mL) of Ca Standard Solution Titrated
Amount (mmoles) Ca
Amount (mmoles) EDTA reacted
Volume (mL) of the EDTA solution delivered
Molarity of EDTA solution
Average Molarity of EDTA
Deviation
Standard Deviation
Coefficient of Variation (%RSD)
Trial 1
0.009884
25.00
0.2471
0.2471
21.70
0.01139
0.01146
-0.00007
0.00008
0.70
Trial 2
25.00
0.2471
0.2471
21.60
0.01144
-0.00002
Trial 3
25.00
0.2471
0.2471
21.40
0.01155
+0.00009

Transcribed Image Text:Table 1A. Preparation of Ca²+ Standard Solution
MMcacos = 100.09g/mol
mass CaCO3 (g)
Amount(mmol) Ca-Amount(mmoles) CaCO3
Volume (mL) of Ca Standard Solution
Molarity of Ca Standard Solution
0.2473
2.471
250
0.009884
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Does that mean that Average molarity of EDTA standard solution = Average molarity of EDTA? Will this always be the case?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning