Draw each plane in the x, y, and z axes on its own, with full labels for the given dimensions on each. b. Draw the unit cell to scale (i.e., proportional to the given dimensions). Label the axial lengths and axial angles with full names. c. Find out which crystal system the unit cell is part of.
Draw each plane in the x, y, and z axes on its own, with full labels for the given dimensions on each. b. Draw the unit cell to scale (i.e., proportional to the given dimensions). Label the axial lengths and axial angles with full names. c. Find out which crystal system the unit cell is part of.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.36E: The first two signals from a powdered sample has X rays (=1.47742A) diffracted at angles of 13.48...
Related questions
Question
a. Draw each plane in the x, y, and z axes on its own, with full labels for the given dimensions on each.
b. Draw the unit cell to scale (i.e., proportional to the given dimensions). Label the axial lengths and axial angles with full names.
c. Find out which crystal system the unit cell is part of.
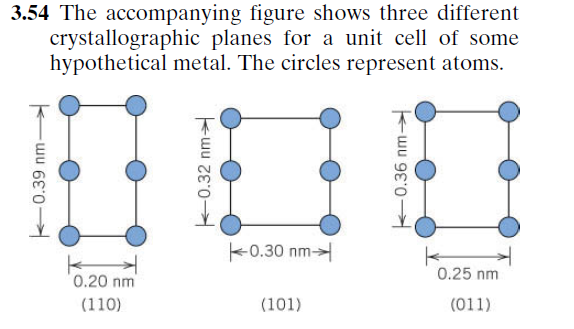
Transcribed Image Text:3.54 The accompanying figure shows three different
crystallographic planes for a unit cell of some
hypothetical metal. The circles represent atoms.
k0.30 nm>
0.25 nm
0.20 nm
(110)
(101)
(011)
0.39 nm-
0.32 nm
F0.36 nm-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning