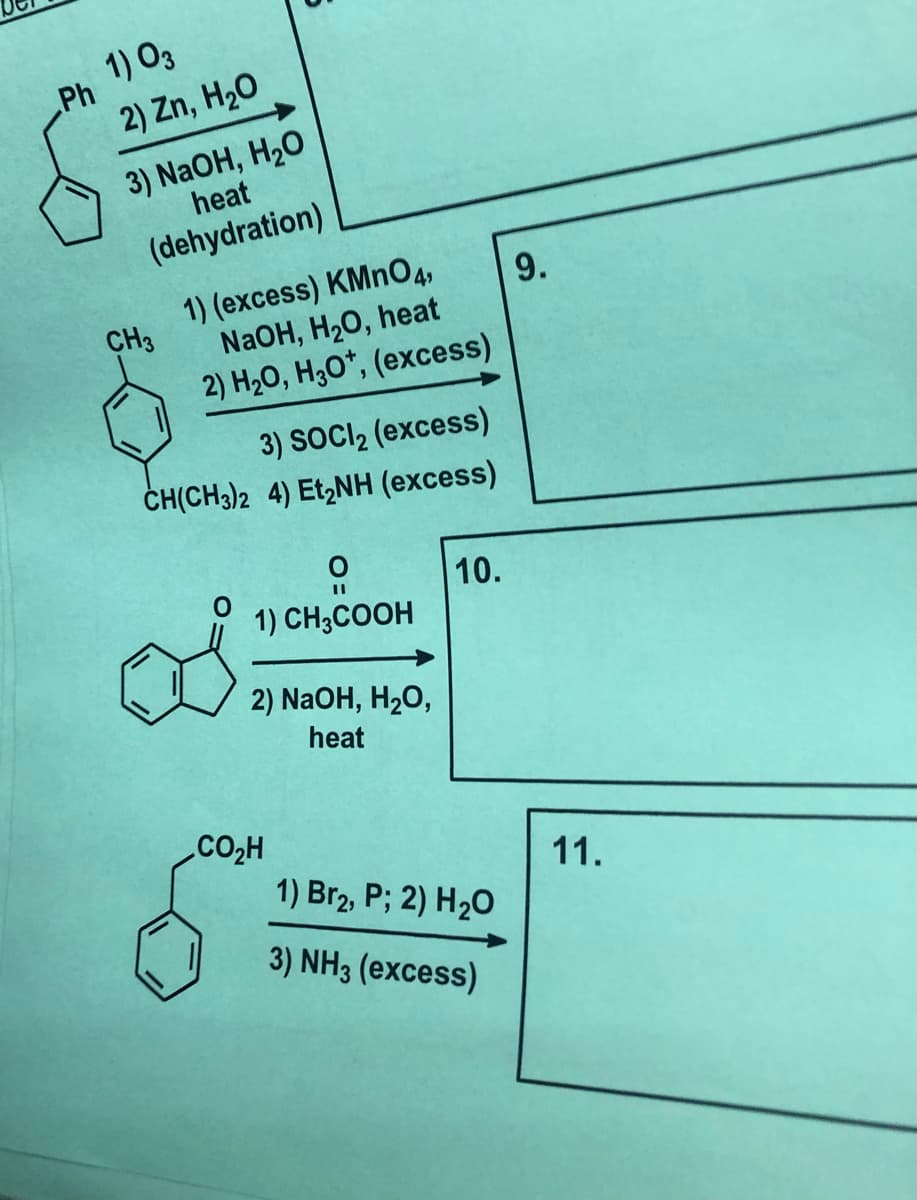
1) O3 Ph 2) Zn, H20 3) NaOH, H2O heat (dehydration) 1) (excess) KMNO4, CH3 NaOH, H2O, heat 9. 2) H20, H3O*, (excess) 3) SOCI2 (excess) ČH(CH3)2 4) Et,NH (excess) 10. 1) CH3COOH 2) NaOH, H2O, heat .CO2H 11. 1) Br2, P; 2) H2O 3) NH3 (excess)
Q: What is the pM in a titration of 20.0 mL of 0.05 M Ca2 with 0.05 M EDTA if the titration is buffered…
A:
Q: For a partar acon at 250C. AG AdrS1 what s as far thes reation n Enter the numerica va fcant…
A: • The values provided in the question are:- i) Standard gibbs free energy change of the reaction,…
Q: Calculate ao ai and a21. for a. picric acid in a solution with a pH of 1.250. b. oxalic acid in a…
A: a.
Q: Provide the appropriate reaagents or product. 1. PdoLn, EtgN,mecN Me -> 2. Na BH, MeOH 3. CI HO…
A:
Q: draw the product
A:
Q: 5. Provide the complete mechanism (arrow, lone pairs, formal charges) for the following…
A: In this question we have to tell the mechanism of the reaction.
Q: 4-oxobutanoic acid : predict number of NMR signals, splitting pattern of signals and integration.
A:
Q: According to the following reaction, how much energy is evolved during the reaction of 32.5 g B2H6…
A:
Q: of Mg coH)2 in 300.6 g of ta Ba water The Ôensity of the final Calculate. punst woynos mut to…
A: " Since you have posted a question with multiple sub-parts , we will solve first three sub-parts for…
Q: d. Write out the equilibrium equation formed at the equivalence point. e. Complete an ICE box using…
A: Solutions- Given data- 0.300 M NaOH solution, 35.0 ml of 0.450 M CH3COOH solution, Acid dissociation…
Q: prepare an M0_diagram quideofatomic obital emergiesBalle d m your Mo, wohich of ithe followsine…
A: Since you have asked multiple questions we will answer the first one for you. To get the remaining…
Q: According to the method, the formal titration of amine nitrogen is Choose one answer: O a.…
A: The correct answer is given below
Q: Question 15 of 60 Submit How many molecules are in 57.2 moles of CH,?
A:
Q: Explain through the concept of bonding why water was found to expand by approximately 9% when it…
A: In Liquid form, water ( H2O) molecules are continuously moving. As a result of this, there is very…
Q: Calculate the enthalpy for the reaction below using standard enthalpies of formation. Show all your…
A: Given, CH4(g) + 3Cl2(g) ➝ 3HCl(g) + CHCl3(l) The standard enthalpy of formation (∆H°f) of all the…
Q: Consider the reaction described by the equation C,H,Br, (aq) + 3I(aq) → C,H,(g) + 2 Br¯(aq) + I5…
A: The speed of the chemical reaction is measured in terms of rate Here we are required to complete…
Q: What happens to the equilibrium point when a disturbance is introduced to a chemical system? O The…
A: The correct answer about equilibrium is given below
Q: Calculate the solubility at 25 °C of CaF, in pure water and in a 0.0110 MNAF solution. You'll find…
A: Given data,Molarity of NaF=0.0110 MKsp of CaF2=3.9×10-11Molar mass of CaF2=78.07 g/mol
Q: At a certain temperature, the equilibrium constant K for the following reaction is 0.010 : Br, (g) +…
A: We have to predict the correct statement about equilibrium
Q: For the electrochemical cell 2 Al(s) + 3 Mn²⁺(aq) ⟶ 2 Al³⁺(aq) + 3 Mn(s) (E° = 0.48 V, [Al³⁺] = 1.0…
A:
Q: CH3OH он Rxn 1 CH,OH Br LOH Rxn 2
A:
Q: What is the pressure exerted by 1.54 g Xe gas at 17.0 °C in a 350-mL flask?
A:
Q: pressure of the gas is increased, while at the same time it is heated to a higher temperature, the…
A:
Q: A sample of helium gas at a pressure of 923 mm Hg and a temperature of 72 °C, occupies a volume of…
A:
Q: For each compound in the table below, decide whether there would be any hydrogen-bonding force…
A: Hydrogen bonding is present between electronegative atom and hydrogen. It may me intermolecular or…
Q: Principles of Chromatography
A: Definition :
Q: Calculate the amount of heat needed to boil 108. g of acetic acid (HCH,CO,), beginning from a…
A:
Q: Which of the following aqueous solutions are good buffer systems? O 0.20 M perchloric acid + 0.21 M…
A: Buffer solutions are of two types : 1.) Acidic buffer : These are made up of weak acid and it's…
Q: Calculate the molar concentration of H2S04 (98.0 g/mol) in a solution that has a specific gravity of…
A: 95 % (w/w) concentration of sulfuric acid states that the 100 g of solution contains 95 g of…
Q: Activity 3 Direction: Match the following processes with their descriptions. 1. Storage a. Some…
A: General chemistry
Q: O Draw a line- angle structure f a compound wh He tellouing charactenstics's I chiral carbon,…
A: IUPAC nomenclature is used for naming the organic compound as recommended by international union of…
Q: The rate of disappearance of HBr in the gas phase reaction 2HBR (g) H2 (g) + Br2 (g) is 0.301 M s-1…
A: ANS. 0.151
Q: 24-33 Predict the product(s) and provide the mechanism for each reaction below. (a) 1. NaN3 2. H20,…
A: Curtius rearrangement : Acyl chloride reaction with NaN3, H2O, heat produces primary amines ( see…
Q: What role does an alkyl group have in the following reactions: SN1 SN2 E1 E2
A:
Q: (Q59) What is the difference between the C-H bond that exists in methane (CH4) versus the bonding…
A: The bonding in simple Compounds and coordination complex is different. In simple Compounds , atoms…
Q: 1. What are some common uses for simple distillation? What are some common uses for fractional…
A:
Q: Dry ice is solid carbon dioxide. Instead of melting, solid carbon dioxide sublimes according to the…
A: Given, Sublimation of carbon dioxide : CO2(s) → CO2(g)
Q: Use the information in the ALEKS Data tab to sort the following chemical species by reducing power.…
A:
Q: Explain why water expand when it freezes. Based the answer on the concept of bonding and include a…
A: water molecule in liquid state are more close and not present in any particular shape or…
Q: The resulting retention volumes for a series of polystyrene standards Molar mass (g/mol) Retention…
A: Given: Molar mass (g/mol) log(Mw) Retention volume (mL) 640000 5.80618 12.14 483000 5.683947…
Q: 5. How many calories are needed to heat 50 g of ice at -17 deg C to steam at 115 deg C? C (ice) =…
A: Given data,Mass of H2O=50 gInitial temperature=-17oCFinal temperature=115oCCice=0.5…
Q: What is the the equilibrium constant (K) for the reaction below, if the reaction mixture initially…
A:
Q: Balance the following redox reaction in acidic solution. Mn" (aq)+Cu (aq) - MnO,(s)+Cu (aq) Mn (aq)…
A: The steps for balancing redox reaction in acidic solution is as follows - 1) Divide the reaction…
Q: Explain the difference ber- a. using the Fischer form
A:
Q: What is the change in enthalpy (∆H) when a 147 g of ice at -23.7°C is heated to a liquid at 66.5°C?…
A:
Q: 13. The speed of a particular automobile is 45 mph (miles per hour). Use the following relationships…
A: In this question we have to convert the unit of speed.
Q: e highest pH?
A:
Q: QUESTION 13 The IUPAC name of the compound CH3CH2COOH is O propanoic acid O butanoic acid O…
A: In this question we have to tell the IUPAC name of the compound.
Q: 80 20 5 Determine the EA for the rate-determining step (step 1) for this reaction coordinate…
A: Minimum energy is required for reactant species to form products is known as activation energy.
Q: MASS SPECTRUM 100 80 60 40 20 0.0+ 0.0 20 40 60 80 100 m/z Rel. Intensity
A: The compound name is 2-pentanone. m/z = 43 corresponds to CH3CO+. Molecular ion at 86. M+1 peak at…
Step by step
Solved in 3 steps with 2 images

- The maximum dose over 24 hours for OTC (over the counter use) of acetaminophen (medication in Tyleonol) is 4,000 mg. Many OTC and prescription medications contain acetaminophen along with other ingredients. Acetaminophen can be found in many cold medicines and in prescription painkillers such as Norco, which also contains the addictive opiod hydrocodone. Keeping track of sources and amount of acetaminophen is very important for your health. Abuse of Norco due to the opiod can also lead to toxic/lethal doses of acetaminophen. There are now strict regulations on the number of tablets of Norco that can be prescribed (7 day maximum per prescription, not refillable without another prescription). A tablet of Norco contains 325 mg of acetaminophen. Thirty tablets are in a week-long prescription post major-surgery to aid in recovery. How many grams of acetaminophen are in the 30 tablets?Using the percent purity calculations, determine the percent yield of synthesis of aspirin. Part I Synthesis of Aspirin Mass of salicylic acid used (g) 2.029g Volume of acetic anhydride used (mL) 5ml Mass of acetic anhydride used (vol. × 1.08 g/mL) 5.4g Mass of aspirin synthesized (g) 3.256g Part II Melting Temperature Data Melting temperature (°C) 133°C Part III Salicylic Acid Standard Stock Solution Initial mass of salicylic acid (g) 0.210g Moles of salicylic acid (mol) 0.0147 mol Initial molarity of salicylic acid (M) 0.724 M Part III Beer’s Law Data for Salicylic Acid Standard Solutions Trial Concentration (M) Absorbance Water (mL) 1 10 0.301 0 2 7.5 0.219 2.5 3 5.0 0.163 5.0 4 2.5 0.074 7.5 Best-fit line equation for the salicylic acid standards Test of the Purity of the Synthesized Aspirin Initial mass of aliquot of product (g)…In an Orsat analysis the % CO2 of the product of the combustion is 20% . If percent excess air is 30% . find the maximum percentage of CO2 attainable.
- Explain why it is problematic to include a constraint such as the following in an LP model for a blending problem: Total octane in gasoline 1 blend $ divided by Barrels of gasoline 1 blended daily which is greater thanor equal to 10The goal is to convince an investor to invest in your company so that you can build a factory to make ester compounds. For example, you could make hexyl pentanoate, and sell it for use in perfumes and make the investor money. You must come up with a compound that you can make and sell. You can use your textbooks and theinternet as resources. You can also access the link: https://jameskennedymonash.wordpress.com/2013/12/13/infographic-table-of-estersand-their-smells/ to look at an infographic of the ester compounds responsible for the smell of different fruits, perfumes and flowers. At the end of your research, you must have the compound table filled outbalancing equations C2H6+O2=CH3COOH+H2O
- H2SO3 = 0.2556 M, Ka1 = 1.6 x 10-2 , Ka2 = 6.4 x 10-8 NaOH = 0.3106 M please show all work all information needed is herePlease show steps and answer accordingly. Thanks!1-Pentanol to 1-bromopentane Chemicals: - 60ml Conc. Sulfuric Acid - 100ml Saturated Sodium bicarbonate - 65ml 1-Pentanol - 78g sodium bromide - Distilled water - 58.42g 1-Bromopentane 1-Pentanol Sodium Bromide Sulfuric Acid 1-Bromopentane Formula C5H12O NaBr H2SO4 C5H11Br MW (g/mol) 88.15 102.894 98.078 151.04 Density (g/mL) 0.811 3.21 1.84 1.218 Boiling point (*C) 138 1,396 337 130 NaBr(aq) + H2SO4(aq) -> NaHSO4(aq) + HBr(aq) CH3(CH2)4OH(aq) + H+ Br- (aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4Br(aq) + H2O(aq) How do I calculate the percent yield and identify the limiting reagent?
- 2I– (aq) + H2O2 (aq) + 2H3O + (aq) → I2 (aq) + 4H2O (l) (slow) C6H8O6 (aq) + 2H2O (l) + I2 (aq) → C6H6O6 (aq) + 2H3O + (aq) + 2I– (aq) (very fast) I2 (aq) + I– (aq) ⇌ I – 3 (aq) I3- (aq) + starch → blue I3- · starch complex (aq) (fast) (a) A 0.100 L solution is prepared with initial concentrations of 4.0 × 10−3 M iodine I2 , 8.0×10−3 M iodide I– , and 5.0×10−3 M ascorbic acid C6H8O6 . After the second reaction goes to completion, what will the molar concentrations of iodide and ascorbic acid in the solution be?There's 1 drink (and you are asked to determine the glucose concentration in the drink in the units of g/100mL. (Why these units? Well, once you have the concentrations in g/100mL you will be able to compare your values with the nutritional values given on the drink bottles’ labels). The sample of the drink was diluted 1/100 (i.e. by a factor of 100). This was an essential step in the method because, without it, the machine used to analyse the glucose concentration (spectrophotometer) would have given an error as the concentration would have been too high for accurate detection. What this means for you is that the dilution factor will need to be taken into consideration in your calculations (remember the aim is to calculate the concentration in the original drink and not in the diluted drink). You measured the concentration of their diluted drink using the spectrophotometer and their results were provided to them in the units mM (millimolar). Glucose Concentration in mM of drink =…There's 1 drink (and you are asked to determine the glucose concentration in the drink in the units of g/100mL. (Why these units? Well, once you have the concentrations in g/100mL you will be able to compare your values with the nutritional values given on the drink bottles’ labels). The sample of the drink was diluted 1/100 (i.e. by a factor of 100). This was an essential step in the method because, without it, the machine used to analyse the glucose concentration (spectrophotometer) would have given an error as the concentration would have been too high for accurate detection. What this means for you is that the dilution factor will need to be taken into consideration in your calculations (remember the aim is to calculate the concentration in the original drink and not in the diluted drink). You measured the concentration of their diluted drink using the spectrophotometer and their results were provided to them in the units mM (millimolar). Glucose Concentration in mM of drink =…



