Draw the Lewis structure of HCIO, (by following the octet rule on all atoms) and then choose the appropriate pair of molecular geometries of the two central atoms. Your answer choice is independent of the orientation of your drawn structure. CI-O H A) linear / planar B) pyramidal/trigonal pyramidal C) trigonal pyramidal / bent (109.5") D) linear/trigonal E) bent (109.5") / linear O:
Draw the Lewis structure of HCIO, (by following the octet rule on all atoms) and then choose the appropriate pair of molecular geometries of the two central atoms. Your answer choice is independent of the orientation of your drawn structure. CI-O H A) linear / planar B) pyramidal/trigonal pyramidal C) trigonal pyramidal / bent (109.5") D) linear/trigonal E) bent (109.5") / linear O:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 62P: For each of the following molecules or molecular ions, give the steric number, sketch and name the...
Related questions
Question
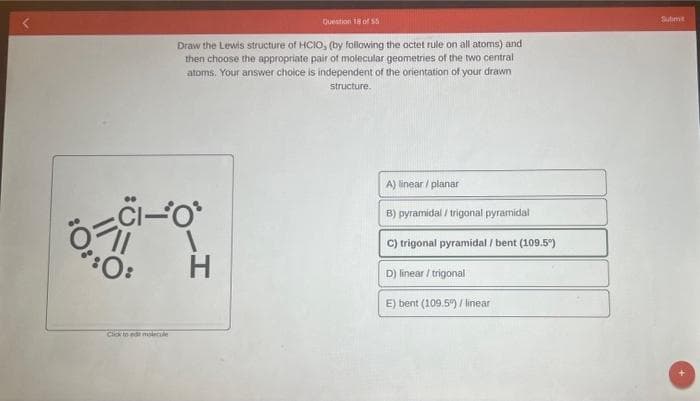
Transcribed Image Text:Question 18 of 55
Draw the Lewis structure of HCIO, (by following the octet rule on all atoms) and
then choose the appropriate pair of molecular geometries of the two central
atoms. Your answer choice is independent of the orientation of your drawn
structure.
CI-O
:0:
H
A) linear/planar
B) pyramidal/trigonal pyramidal
C) trigonal pyramidal / bent (109.5°)
D) linear/trigonal
E) bent (109.5%) / linear
Click to edit molecule
Subme
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning