Draw the sigma complex for the formation of the bromonium electrophile with proper resonance forms for the meta bromination of toluene and for the para bromination of toluene. Which resonance structure places the (+) next to the methyl group? Will either one (i.e. para and meta sigma complex) yield a fourth resonance structure? Given that alkyl groups such as CH3 are electron rich (i.e. no attached electronegative atoms), which sigma complex in part (A) would be more stable?
Carbohydrates
Carbohydrates are the organic compounds that are obtained in foods and living matters in the shape of sugars, cellulose, and starch. The general formula of carbohydrates is Cn(H2O)2. The ratio of H and O present in carbohydrates is identical to water.
Starch
Starch is a polysaccharide carbohydrate that belongs to the category of polysaccharide carbohydrates.
Mutarotation
The rotation of a particular structure of the chiral compound because of the epimerization is called mutarotation. It is the repercussion of the ring chain tautomerism. In terms of glucose, this can be defined as the modification in the equilibrium of the α- and β- glucose anomers upon its dissolution in the solvent water. This process is usually seen in the chemistry of carbohydrates.
L Sugar
A chemical compound that is represented with a molecular formula C6H12O6 is called L-(-) sugar. At the carbon’s 5th position, the hydroxyl group is placed to the compound’s left and therefore the sugar is represented as L(-)-sugar. It is capable of rotating the polarized light’s plane in the direction anticlockwise. L isomers are one of the 2 isomers formed by the configurational stereochemistry of the carbohydrates.
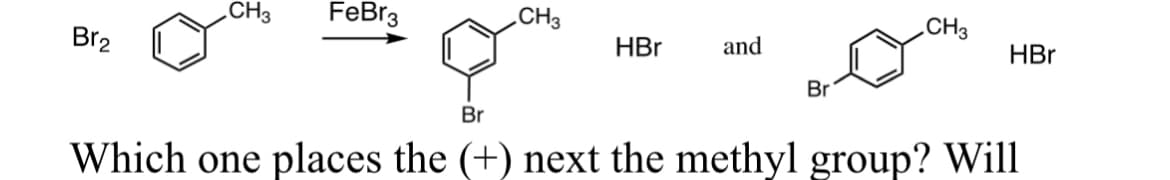
Given that,
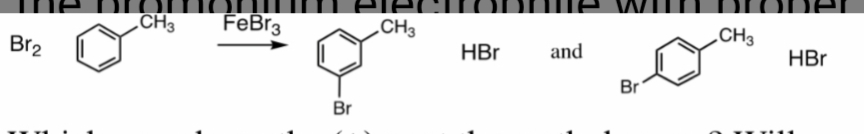
We have to draw the structure of the Sigma complex form from the above two products.
Also, we have to tell which one will generate the positive charge next two the methyl group.
And, also we have to determine which one is the most stable sigma complex.
Step by step
Solved in 3 steps with 4 images







