Q: 1. Give a synthetic route (hint: protecting group). OH
A:
Q: Why an anhydride is less reactive than an acyl chloride?
A: The reactivity of acyl chloride and acid anhydride occurs due to the electrophilicity of carbonyl…
Q: I have questions about p-nitroaniline: Why we are not starting from aniline but from acetanilide…
A: Reference: Self Introduction: Amino group shows very high reactivity towards a wide variety of…
Q: likely to form when reacting Ethyl amine and excess ethyl
A: Organic reactions are those in which organic reactant react to form organic products. We have to…
Q: Give an equation for the complete hydrogenation of trilinolein using an excess ofhydrogen. Name the…
A:
Q: Kevlar®, a polymeric aromatic amide, is synthesized from the monomers p-phenylenediamine (left) and…
A: (a) Lewis acid is a compound that can accept or share the electron pair from another compound. Lewis…
Q: What makes chlorosulfonic acid a good electrophile?
A: The compound given is chlorosulfonic acid i.e HSO3Cl.
Q: Explain why acetyl chloride reacts faster with water than acetic anhydride does?
A: For the given two compounds, i.e. The relative reactivity of these compounds with water has to be…
Q: Label the cis-[Dichlorobis(ethylenediamine)cobalt(III)] Chloride product
A: The molecular formula of - Dichlorobis(ethylenediamine)cobalt(III)] Chloride is: [CoCl2 (en)2 ]+
Q: Why an anhydride is more reactive than a carboxylic acid?
A: There are many carboxylic acid derivatives present and acid anhydride is now of those derivates. The…
Q: Provide a reasonable synthetic route
A:
Q: d) .F
A: benzene undergoes electrophilic substitution reaction due to pi-electron cloud over the whole…
Q: Define Reduction of Nitriles ?
A: Reduction Reduction means addition of hydrogen Or remove of oxygen Nitrite - compound containing…
Q: Describe (using epoxides.- an appropriate figure) an additional way to prepare an enantioenriched
A: Interpretation: To describe a way to prepare an enantioenriched epoxides.
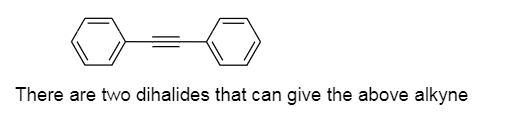
Q: Draw the structure of a dihalide that could be used to prepare attachedalkyne. There may be more…
A:
Q: What compounds can be best combined with arendiazonium salts? Why?
A: Arene diazonium salts are one of the combinations of organic and inorganic components. It is formed…
Q: What will be the result for Pentan-2-one under DNP test, Tollen's test, and iodoform test.
A: *When ketones react with 2,4-DNP , they give hydrazone. * When ketones react with Tollen's reagent,…
Q: Give another dye that is made using diazonium salt chemistry/coupling reaction. Draw the dye as…
A: 1. An azo dye structure must have R-N=N-R' 2. Here R and R' must be a aromatic compound. 3. There…
Q: Friedel- Crafts acylation. How can sulfuric acid be used as an
A: RCOOH+H2SO4>>>>>>RCO+ +H2O +HSO4-
Q: Draw the benzoic acid structure and explain which products will be formed as a result of the…
A: Benzoic acid is an aromatic compound (carboxyl group attached to benzene ring) and aromatic…
Q: What is the electrophoresis in the aromatic nitration ?
A: We have to find which electrophile is correct for the nitration
Q: Outline two different ways that butan-2-one can be prepared from a nitrile and a Grignard reagent.
A: The reaction of the Grignard reagent with a nitrile compound gives imine which on hydrolysis…
Q: explain the SODIUM BOROHYDRIDE REDUCTION
A: In reduction reaction, hydrogen is added to the reactant. Sodium borohydride adds hydrogen to some…
Q: Why is an alkylamine more basic than ammononia?
A: Because of electron delivering nature, the alkyl gathering (R) pushes electrons towards nitrogen in…
Q: Why are the cyanates and sulfonates not as toxic as the other compounds of cyanide?
A: Cyanide is the CN-particle, and as a harm it is generally controlled as one of the three mixtures…
Q: Which of the following is suitable to use when forming grignard reagent
A: Grignard reagent has general formula of R/Ar-MgX , where R is an alkyl group and Ar is an aromatic…
Q: What products would form from a reaction between Potassium tert-butoxide in THF and the following…
A:
Q: Explain how much excess acid was used to make the "nitrous acid" HONO that will make the diazo salt…
A: The functional groups associated with a molecule are key molecular components that are responsible…
Q: Provide another dye that is made using diazonium salt chemistry/coupling reaction. Draw the dye as…
A: Azo dye:- These dyes are characterized by the presence of azo groups. The azo groups coupled with…
Q: Identify the following structure if it will give a positive result to Fehling's Test
A: Fehling's test is used for identification of aldehyde and ketone fo not give Fehling's test.
Q: NH2 In which active solvent will you choose to extract from chloroform (CHC3)?
A: The separation of compound from the mixture on basis of their polarity is known as differential…
Q: What do you mean by Clemmensen reduction ?
A: Clemmensen reduction: Clemmensen reduction is a reaction in which carbonyl group of aldehydes and…
Q: 3. Give another dye that is made using diazonium salt chemistry/coupling reaction. Draw the dye as…
A: Azo dye:- These dyes are characterized by the presence of azo groups. The azo groups coupled with…
Q: Please explain the Chemistry behind the synthesis process of Sulfonamides.
A:
Q: Enolates are formed by deprotonation of an α-carbon hydrogen. Answer the following questions about…
A: in this given molecule alpha carbon is adjacent carbon of carbonyl group, that is at the ring…
Q: Why is it so important to use only dry glassware and solvents when you hope to perform a…
A: Solution (a) when we proceed any reaction we must have to ensure that reactant must have no impurity…
Q: A greener alternative to bromination with elemental bromine is the reaction of the acetanilide with…
A: Ammonium ceric nitrate is an inorganic compound. It's molecular formula is (NH4)2Ce(NO3)6. It is…
Q: If phthalic anhydride is converted into phthalic acid how it will be again converted into anhydride…
A: How phthalic acid will be again converted into anhydride form has to be given,
Q: How the reduction of disulfides to thiols could be done.
A: The reduction of disulfides to thiols could be done has to be determined.
Q: Explain the Nitration ?
A: Nitration is the addition of nitrogen to the the compound.
Q: How would this compound react in ninhydrin test?
A:
Q: What experimental means may be used to drive an esterification towards completion?
A: Esterification is the process of forming an ester from the two different reactants namely carboxylic…
Q: Explain the Sodium Bisulfite Test for aldehydes (positive and negative result)
A: When Aldehydes react with sodium bisulfite (NaHSO3), then a water soluble crystalline solid…
Q: What starting materials are needed to synthesize attached azo compound?
A:
Draw the structure of a dihalide that could be used to prepare attache dalkyne. There may be more than one possible dihalide.

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- what would be the product and provide the arrow pushing mechanism in each step??Explain the Nitration ?Answer the following parts of the question about Azo dyes:a) Why are the azo compounds synthesized colored? b) Explain the difference between ingrain dyes and direct dyes.c) Also What precautions should be taken when working with diazonium salts and why?
- Why is an alkylamine more basic than ammononia?Fiskesjo, is one of the pioneers of allium test. Diagram his procedure and a recently conducted study and differentiate it.Explain why the heat of neutralization involving acetic acid and hydroxidesodium is lower than the value found in the neutralization reaction involving hydrochloric acid and the sodium hydroxide.

