Draw the two possible chair conformations of the (+)- B- Galactose molecule shown below, using the axial and equatorial bonds provided. Show all substituents and hydrogens. 4. CH-ОН HO ОН ОН CH2OH CHON OH HO OH HO OH HO H OH OH Conformation # 1 Conformation # 2 5. With respect to the previous problem # 4, and your two chair conformations of (+)- B- Galactose.. A single (-OH -- H) 1,3-diaxial steric strain interaction is 2.1 kJ/mole, and a single (-CH2OH -- H) 1,3-diaxial steric strain interaction is 4.0 kJ/mole, and finally a single (-CH2OH steric strain interaction is 4.6 kJ/mole. OH) 1,3-diaxial Determine and Show Calculations for the total 1,3-diaxial steric strain contained in your a) conformations # 1 and # 2. b) Indicate which of your chair conformations is the most stable. Conformation # 1 Conformation # 2 3 Hr
Draw the two possible chair conformations of the (+)- B- Galactose molecule shown below, using the axial and equatorial bonds provided. Show all substituents and hydrogens. 4. CH-ОН HO ОН ОН CH2OH CHON OH HO OH HO OH HO H OH OH Conformation # 1 Conformation # 2 5. With respect to the previous problem # 4, and your two chair conformations of (+)- B- Galactose.. A single (-OH -- H) 1,3-diaxial steric strain interaction is 2.1 kJ/mole, and a single (-CH2OH -- H) 1,3-diaxial steric strain interaction is 4.0 kJ/mole, and finally a single (-CH2OH steric strain interaction is 4.6 kJ/mole. OH) 1,3-diaxial Determine and Show Calculations for the total 1,3-diaxial steric strain contained in your a) conformations # 1 and # 2. b) Indicate which of your chair conformations is the most stable. Conformation # 1 Conformation # 2 3 Hr
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter16: Aldehydes And Ketones
Section: Chapter Questions
Problem 16.67P: Treatment of -D-glucose with methanol in the presence of an acid catalyst converts it into a mixture...
Related questions
Question
#5
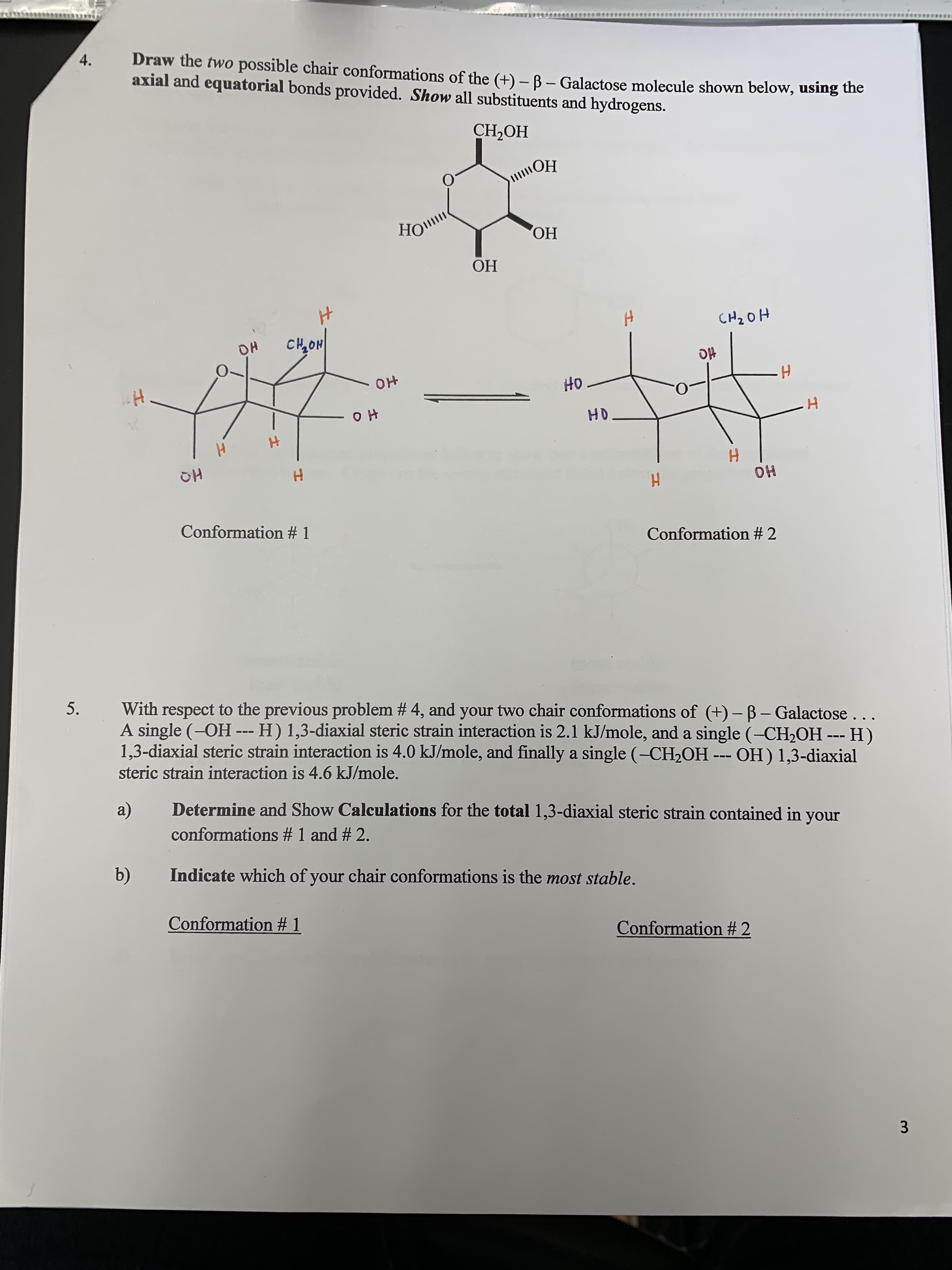
Transcribed Image Text:Draw the two possible chair conformations of the (+)- B- Galactose molecule shown below, using the
axial and equatorial bonds provided. Show all substituents and hydrogens.
4.
CH-ОН
HO
ОН
ОН
CH2OH
CHON
OH
HO
OH
HO
OH
HO
H
OH
OH
Conformation # 1
Conformation # 2
5.
With respect to the previous problem # 4, and your two chair conformations of (+)- B- Galactose..
A single (-OH -- H) 1,3-diaxial steric strain interaction is 2.1 kJ/mole, and a single (-CH2OH -- H)
1,3-diaxial steric strain interaction is 4.0 kJ/mole, and finally a single (-CH2OH
steric strain interaction is 4.6 kJ/mole.
OH) 1,3-diaxial
Determine and Show Calculations for the total 1,3-diaxial steric strain contained in your
a)
conformations # 1 and # 2.
b)
Indicate which of your chair conformations is the most stable.
Conformation # 1
Conformation # 2
3
Hr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning