Draw two Lewis structures of the vinyl acetate. Between these structures, identify and label areas of the specific type of intermolecular forces (IM forces) present. Pls draw like this example below H-bonding dispersion Briefly describe these IM forces (each type, its' relative strength) Describe the effect IM forces on the vinyl acetate molecule's: •standard state "vapor pressure (discuss this qualitatively: Iow or high and why...) •melting point and boiling point (again, high or low and why...)
Draw two Lewis structures of the vinyl acetate. Between these structures, identify and label areas of the specific type of intermolecular forces (IM forces) present. Pls draw like this example below H-bonding dispersion Briefly describe these IM forces (each type, its' relative strength) Describe the effect IM forces on the vinyl acetate molecule's: •standard state "vapor pressure (discuss this qualitatively: Iow or high and why...) •melting point and boiling point (again, high or low and why...)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 22E: The test tubes shown here contain equal amounts of the specified motor oils. Identical metal spheres...
Related questions
Question
pls immediately help Thank you
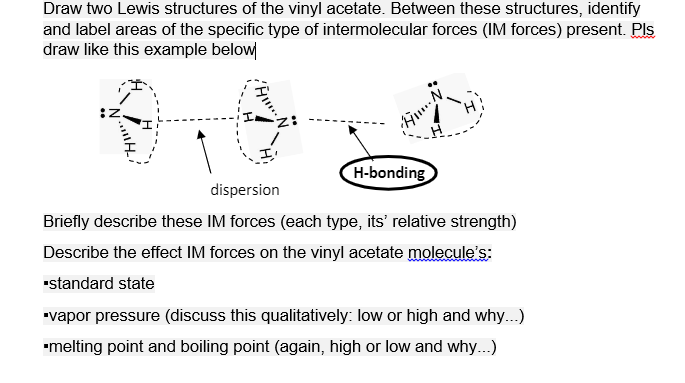
Transcribed Image Text:Draw two Lewis structures of the vinyl acetate. Between these structures, identify
and label areas of the specific type of intermolecular forces (IM forces) present. Pls
draw like this example below
H-bonding
dispersion
Briefly describe these IM forces (each type, its' relative strength)
Describe the effect IM forces on the vinyl acetate molecule's:
"standard state
"vapor pressure (discuss this qualitatively: low or high and why...)
"melting point and boiling point (again, high or low and why...)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning