Dry air (0% humidity) is approximately 78% N₂, 21% O₂, and 1% Ar by moles. Calculate the molar mass and density of dry air at 0 °C and 100 kPa. Assume ideal behavior and use two significant figures in your answers. molar mass= I density= ....pdf x10 TOOLS Typical outdoor air also has water vapor, H₂O(g), mixed in with the other gases. Consider how the molar mass and density of humid air compare to dry air. The molar mass of humid air is The density of humid air is Sample Paper_P....pdf the molar mass of dry air. g/mol the density of dry air. g/L Show All X
Dry air (0% humidity) is approximately 78% N₂, 21% O₂, and 1% Ar by moles. Calculate the molar mass and density of dry air at 0 °C and 100 kPa. Assume ideal behavior and use two significant figures in your answers. molar mass= I density= ....pdf x10 TOOLS Typical outdoor air also has water vapor, H₂O(g), mixed in with the other gases. Consider how the molar mass and density of humid air compare to dry air. The molar mass of humid air is The density of humid air is Sample Paper_P....pdf the molar mass of dry air. g/mol the density of dry air. g/L Show All X
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.105QP: A glass tumbler containing 243 cm3 of air at 1.00 102 kPa (the barometric pressure) and 20C is...
Related questions
Question
8
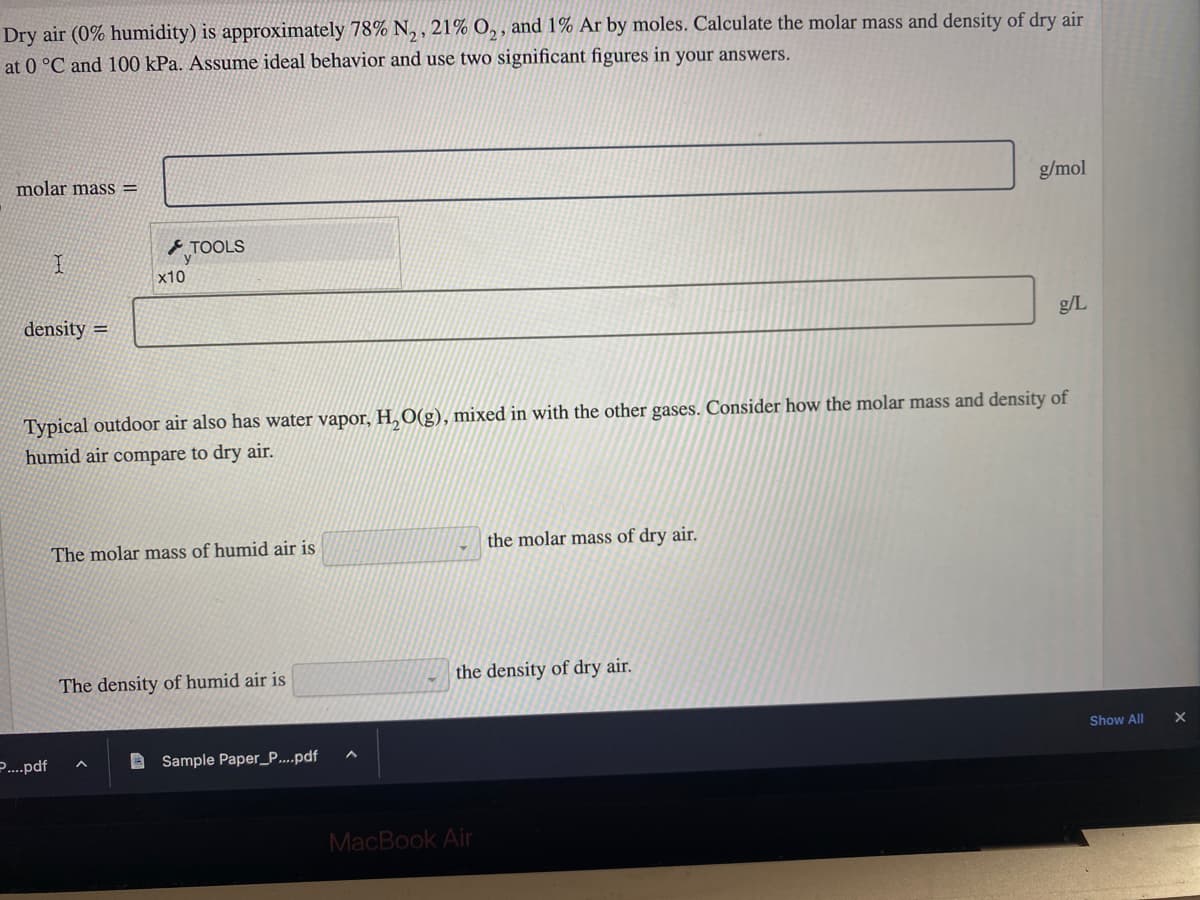
Transcribed Image Text:Dry air (0% humidity) is approximately 78% N₂, 21% O₂, and 1% Ar by moles. Calculate the molar mass and density of dry air
at 0 °C and 100 kPa. Assume ideal behavior and use two significant figures in your answers.
molar mass=
I
density=
P....pdf
8
x10
TOOLS
Typical outdoor air also has water vapor, H₂O(g), mixed in with the other gases. Consider how the molar mass and density of
humid air compare to dry air.
The molar mass of humid air is
The density of humid air is
Sample Paper_P....pdf
A
the molar mass of dry air.
the density of dry air.
MacBook Air
g/mol
g/L
Show All
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning