Due to the small size of the subatomic particles, the only way scientists learned to probe themis to use photons. Based on the implications of the particle-like behavior of photons, this greatly changed how scientists thought about their experiments. In order to obtain more precise measurements, scientists had continued to use higher energy photons. Due to the larger momentum associated with higher energy photons, this meant that the measurements would more greatly alter the momentum of the subatomic particle after the measurement was made. For a particular atom, the electron is estimated to travel at a speed of 3.46 X 10° km/s with an error of 2.76%, How uncertain would the electron's position measurement be in units of nanometers? Round your final answer to two significant figures.
Due to the small size of the subatomic particles, the only way scientists learned to probe themis to use photons. Based on the implications of the particle-like behavior of photons, this greatly changed how scientists thought about their experiments. In order to obtain more precise measurements, scientists had continued to use higher energy photons. Due to the larger momentum associated with higher energy photons, this meant that the measurements would more greatly alter the momentum of the subatomic particle after the measurement was made. For a particular atom, the electron is estimated to travel at a speed of 3.46 X 10° km/s with an error of 2.76%, How uncertain would the electron's position measurement be in units of nanometers? Round your final answer to two significant figures.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 100AP
Related questions
Question
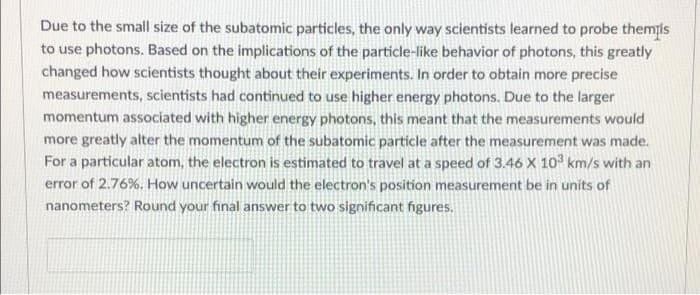
Transcribed Image Text:Due to the small size of the subatomic particles, the only way scientists learned to probe themis
to use photons. Based on the implications of the particle-like behavior of photons, this greatly
changed how scientists thought about their experiments. In order to obtain more precise
measurements, scientists had continued to use higher energy photons. Due to the larger
momentum associated with higher energy photons, this meant that the measurements would
more greatly alter the momentum of the subatomic particle after the measurement was made.
For a particular atom, the electron is estimated to travel at a speed of 3.46 X 10 km/s with an
error of 2.76%. How uncertain would the electron's position measurement be in units of
nanometers? Round your final answer to two significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
