During a bout with the flu an 80 kg man ran a fever of 39.0°C (102.2°F) instead of the normal body temperature of 37.0°C (98.6°F). Assuming that the human body is mostly water, how much heat is required to raise his temperature by that amount? IDENTIFY and SET UP This problem uses the relationship among heat (the target variable), mass, specific heat, and tem- perature change. We use Eq. (17.13) to determine the required heat Q. with m = 80 kg. c = 4190 J/kg K (for water), and AT = 39.0°C - 37.0°C = 2.0 C = 2.0 K. EXECUTE From Eq. (17.13), Q = mc AT = (80 kg)(4190 J/kg · K)(2.0 K) = 6.7 x 10°J If the person in the example above is a Terminator machine that is composed of 99.99% Aluminum. How much energy is required to raise his temperature on the said problem?
During a bout with the flu an 80 kg man ran a fever of 39.0°C (102.2°F) instead of the normal body temperature of 37.0°C (98.6°F). Assuming that the human body is mostly water, how much heat is required to raise his temperature by that amount? IDENTIFY and SET UP This problem uses the relationship among heat (the target variable), mass, specific heat, and tem- perature change. We use Eq. (17.13) to determine the required heat Q. with m = 80 kg. c = 4190 J/kg K (for water), and AT = 39.0°C - 37.0°C = 2.0 C = 2.0 K. EXECUTE From Eq. (17.13), Q = mc AT = (80 kg)(4190 J/kg · K)(2.0 K) = 6.7 x 10°J If the person in the example above is a Terminator machine that is composed of 99.99% Aluminum. How much energy is required to raise his temperature on the said problem?
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 18PE: Most automobiles have a coolant reservoir to catch radiator fluid than may overflow when 1he engine...
Related questions
Question
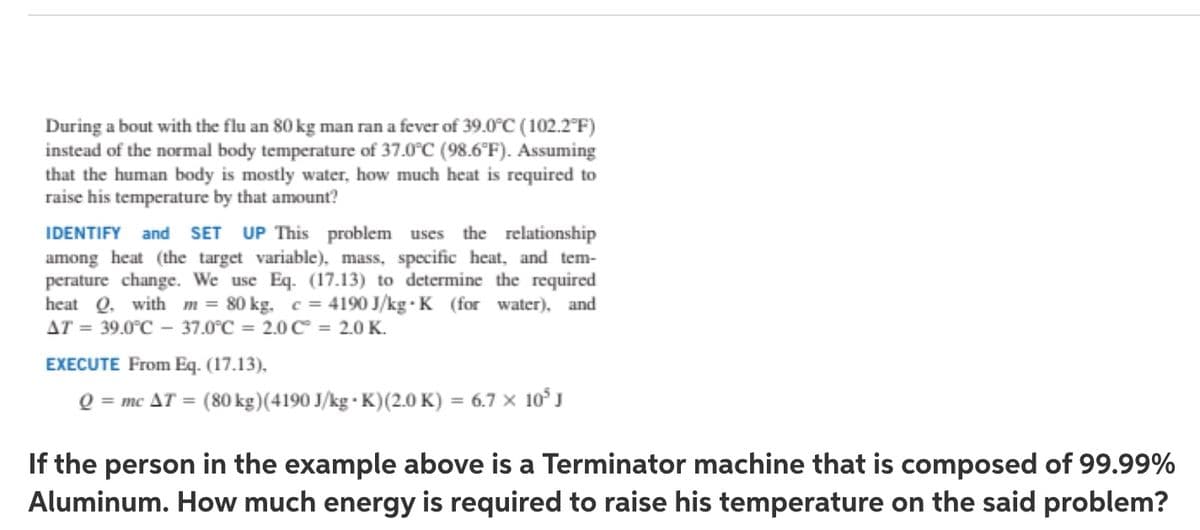
Transcribed Image Text:During a bout with the flu an 80 kg man ran a fever of 39.0C (102.2°F)
instead of the normal body temperature of 37.0°C (98.6°F). Assuming
that the human body is mostly water, how much heat is required to
raise his temperature by that amount?
the relationship
IDENTIFY and SET UP This problem uses
among heat (the target variable), mass, specific heat, and tem-
perature change. We use Eq. (17.13) to determine the required
heat Q. with m = 80 kg. c = 4190 J/kg · K (for water), and
AT = 39.0°C - 37.0°C = 2.0C = 2.0 K.
%3D
EXECUTE From Eq. (17.13),
Q = mc AT = (80 kg)(4190 J/kg · K)(2.0 K) = 6.7 × 10°J
If the person in the example above is a Terminator machine that is composed of 99.99%
Aluminum. How much energy is required to raise his temperature on the said problem?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning