Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question
100%
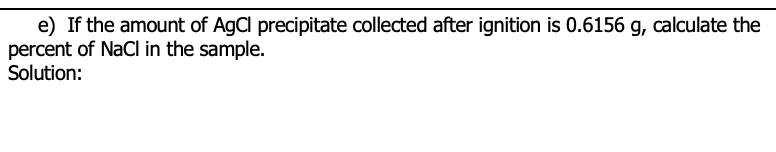
Transcribed Image Text:e) If the amount of AgCl precipitate collected after ignition is 0.6156 g, calculate the
percent of NaCl in the sample.
Solution:

Transcribed Image Text:1. In the Gravimetric Analysis of Chloride by Precipitation, a student collected the following
data:
mass of weighing bottle + NaC sample (g) = 15.0081
mass weighing bottle empty (g)
= 14.7514
a) Calculate the mass of NaCl sample in grams.
Solution:
b) Write the balanced chemical reaction between NaCl and AGNO3.
Solution:
c) If the sodium chloride is to be precipitated with 0.10 M AGNO3, calculate how much is
theoretically required to precipitate all the chloride in the sample?
Solution:
d) If the procedure, requires 10% excess of the calculated volume, what must be the
total volume added to the sample solution?
Solution:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:e) If the amount of AgCl precipitate collected after ignition is 0.6156 g, calculate the
percent of NaCl in the sample.
Solution:

Transcribed Image Text:d) If the procedure, requires 10% excess of the calculated volume, what must be the
total volume added to the sample solution?
Solution:
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning