E) In anoxic conditions (e.g. Ен= -0.3 V and pH=8) trace metals form very insoluble sulfides. Would it make sense to treat water to anoxic conditions to eliminate dissolved Hg? 1. What is the balance of sulfates/sulfides species? 2. Make a Pourbaix diagram for sulfate/sulfide species. 3. What other minerals can precipitate? (Hint: use the concentration of sulfides computed in the previous step, recompile the matrix using S²- and without e¯ and check for precipitation Hg + S minerals)
E) In anoxic conditions (e.g. Ен= -0.3 V and pH=8) trace metals form very insoluble sulfides. Would it make sense to treat water to anoxic conditions to eliminate dissolved Hg? 1. What is the balance of sulfates/sulfides species? 2. Make a Pourbaix diagram for sulfate/sulfide species. 3. What other minerals can precipitate? (Hint: use the concentration of sulfides computed in the previous step, recompile the matrix using S²- and without e¯ and check for precipitation Hg + S minerals)
Chapter6: The Systematic Approach To Equilibria: Solving Many Equations
Section: Chapter Questions
Problem 12P
Related questions
Question
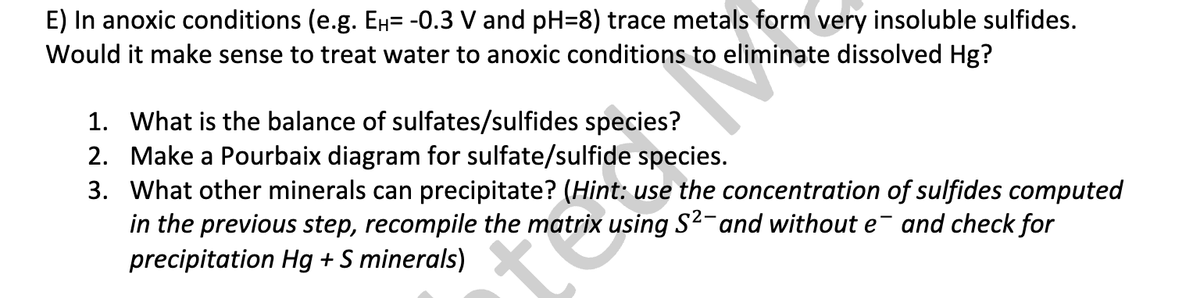
Transcribed Image Text:E) In anoxic conditions (e.g. Ен= -0.3 V and pH=8) trace metals form very insoluble sulfides.
Would it make sense to treat water to anoxic conditions to eliminate dissolved Hg?
1. What is the balance of sulfates/sulfides species?
2. Make a Pourbaix diagram for sulfate/sulfide species.
3. What other minerals can precipitate? (Hint: use the concentration of sulfides computed
in the previous step, recompile the matrix using S²- and without e¯ and check for
precipitation Hg + S minerals)
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you



