e the model of an ethanol molecule to answer the question. H H H-C-C-0- H Which statement describes the hydrogen bonds in an ethanol molecule? All six hydrogen atoms are capable of forming hydrogen bonds. A dipole forms between the hydrogen and oxygen atoms, but not between the hydrogen and carbon atoms. O Only the hydrogen atoms attached to the carbon atoms are capable of forming hydrogen bonds. A dipole does not form between the hydrogen and oxygen atoms. O All six hydrogen atoms are capable of forming hydrogen bonds. A dipole forms between the hydrogen atoms and the carbon or oxygen atoms. O Only the hydrogen atom attached to the oxygen atom is capable of forming hydrogen bonds. A dipole does not form between the hydrogen and carbon atoms.
e the model of an ethanol molecule to answer the question. H H H-C-C-0- H Which statement describes the hydrogen bonds in an ethanol molecule? All six hydrogen atoms are capable of forming hydrogen bonds. A dipole forms between the hydrogen and oxygen atoms, but not between the hydrogen and carbon atoms. O Only the hydrogen atoms attached to the carbon atoms are capable of forming hydrogen bonds. A dipole does not form between the hydrogen and oxygen atoms. O All six hydrogen atoms are capable of forming hydrogen bonds. A dipole forms between the hydrogen atoms and the carbon or oxygen atoms. O Only the hydrogen atom attached to the oxygen atom is capable of forming hydrogen bonds. A dipole does not form between the hydrogen and carbon atoms.
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 18STP
Related questions
Question
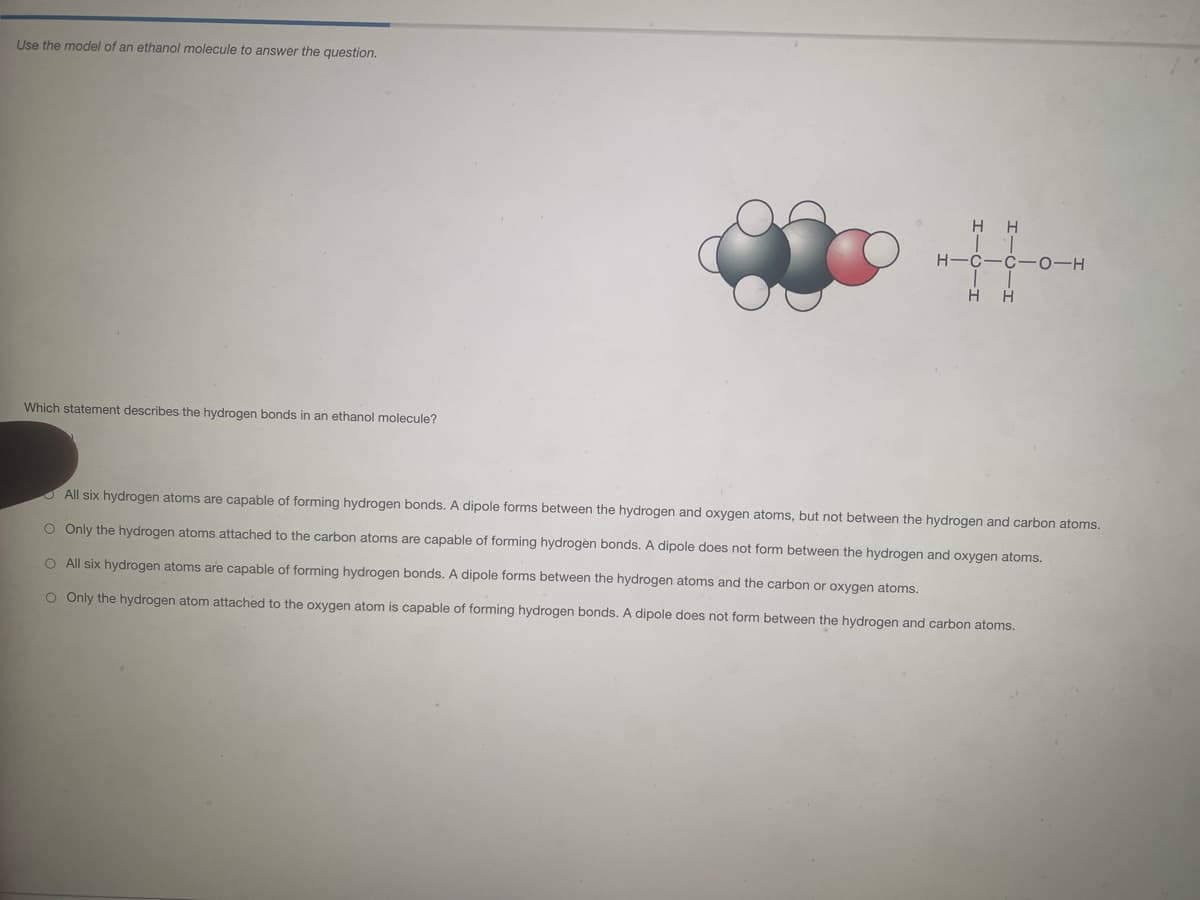
Transcribed Image Text:Use the model of an ethanol molecule to answer the question.
H H
H-C-C- 0-H
H H
Which statement describes the hydrogen bonds in an ethanol molecule?
O All six hydrogen atoms are capable of forming hydrogen bonds. A dipole forms between the hydrogen and oxygen atoms, but not between the hydrogen and carbon atoms.
O Only the hydrogen atoms attached to the carbon atoms are capable of forming hydrogen bonds. A dipole does not form between the hydrogen and oxygen atoms.
O All six hydrogen atoms are capable of forming hydrogen bonds. A dipole forms between the hydrogen atoms and the carbon or oxygen atoms.
O Only the hydrogen atom attached to the oxygen atom is capable of forming hydrogen bonds. A dipole does not form between the hydrogen and carbon atoms.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning



Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning