Q: What is the molar mass of an unknown gas with a density of 2.00 g/L at 1.00 atm and 25.0 °C?
A:
Q: Write the balanced nuclear equation for each of the processes described below. 1. beta emission of…
A:
Q: Which of the following statements is true, given the picture provided. a.Yellow dye is more polar…
A: The given picture is an representative example of the thin layer chromatograpy with various coloured…
Q: 1. Given, for a reaction: Cu (aq) + Fe(s)¬Cu(s) + Fe* (aq) E = 0.78 V Using Nernst equation…
A: Given the electrochemical reaction, Cu2+(aq)+Fe(s)→Cu(s)+Fe2+(aq)Eocell=0.78V Nernst equation is…
Q: When this alkene is subjected to the conditions shown, two equivalents of a single product is…
A: Let's find the product of the given reaction in the question which is 2 equivalent.
Q: Consider the reaction below. which formula represents a conjugate acid-base pair hso3-(aq)+ hf (aq)…
A: Consider the reaction below. which formula represents a conjugate acid-base pair ---
Q: Do you think that the formation of [CoCl4]2-(aq) is endothermic or exothermic? Explain.
A:
Q: The following five beakers, each containing a solution of sodium chloride (NaC1, also known as table…
A:
Q: Ammonia (NH,) chemically reacts with oxygen gas (0,) to produce nitric oxide (NO) and water (H,0).…
A: The amount of Nitric oxide produced is given below
Q: How many moles of particles would result from 71.6 grams of cesium nitrate, CsNO3, dissolved in…
A:
Q: Use the References to access important values if needed for this question. Use the standard…
A: Given reaction: Cu2+ (aq) + Cr2+ (aq) → Cu+ (aq) + Cr3+ (aq) We have to calculate the standard free…
Q: A student weighed out 0.150 g of protein powder and dissolved it in 100 mL of water (Solution 1).…
A:
Q: II. Write the balanced nuclear equation for each of the processes described below. 1. positron…
A: Write the balanced nuclear equation for each of the processess described below-
Q: Calculate the pH of a 0.020 M solution of phenylacetic acid, C6H5CH2COOH. What will be the pH if the…
A:
Q: A fixed amount of helium gas occupies 859 mL at 40.1°C. If this gas is cooled to 16.3°C under…
A: Given :- Initial volume (V1) = 859 mL Initial temperature (T1) = 40.1°C Final temperature (T2) =…
Q: Strontium-90 is one of the products of the fission of uranium-235. This strontium isotope is…
A:
Q: The mass of solute per 100 ml of solution is abbreviated as (m/v). Mass is not technically the same…
A:
Q: Consider the following reaction between iodate and iodide ions. 10, (aq) + 5 I (aq) + 6 H* (aq) → 3…
A: The rate of a reaction can be increased by, • Adding a catalyst - A catalyst decreases the…
Q: ОН Na2Cr207 H2SO4, H2O
A:
Q: Calculate the pH of the following solutions: 0.0035 M HBrO solution (K,=2.5x10) 0.0624 M H,CO,…
A:
Q: Which of the following statements is/are TRUE about the Calorimetry experiment? I. The…
A: following statements are TRUE about the Calorimetry experiment - I. The reactions were kept…
Q: Exercise 16.133 6 of 21 Review I Constants I Periodic Table Common aspirin is acetylsalicylic acid,…
A: Aspirine is a weak acid , so we will use the relation of weak acid's pH , here first we need to…
Q: The value of G° for the conversion of ATP to ADP is -35.6 kJ/mol. The reaction is: ATP(aq) →…
A:
Q: Kindly complete the following nuclear reactions. asap please
A: Nuclear reaction: The given reaction is a nuclear reaction. In the nuclear reaction, one nuclide is…
Q: OH Cro3 HBr -OH H,SO4 а) CH, PPh, b) H;C -CH3 CH;OH H,SO,
A:
Q: The pH of blood is buffered by a mixture of HCO3- (bicarbonate) and H2CO3 (carbonic acid). i. Write…
A: The pH of blood is buffered by a mixture of HCO3- (bicarbonate) and H2CO3 (carbonic acid).i. Write…
Q: C (s) + O2 (e) -100 -200 CaO(s) + CO2 (g) 2 (8) -300 CO 2 (8) CaCO3 (s) -400 Gibbs Free Energy…
A:
Q: retaining (1,4) B-mannosidase H20 HO HO но. OH но. но. но ÓH
A: Concept: Bita-Mannosidase: β-Mannosidase is a lysosomal enzyme which belongs to the glycosyl…
Q: ONLY OH HO NH2
A:
Q: The rate constant of Iodine-131 is 8.66 x 10^-2 per day. 1. What is the half-life of I-131? 2. If…
A: As the unit of rate constant is time-1, it is a first order reaction. For a first order reaction,…
Q: 1.50 moles of a radio nuclide emits 3.09 x 1010 beta particles per second. assumed that each beta…
A: Here 1.5 moles of radio active nuclide emits 3.09×10^10 beta particles per second. We have to…
Q: Be sure to answer all parts. A freshly isolated sample of 90Y was found to have an activity of 2.9 x…
A:
Q: On the basis of Lewis acid and base concept, explain oxidative addition reaction.
A: Oxidative addition reaction: Reaction where addition of ligands to metal centre occurs with…
Q: Which element will have five electrons in its Lewis symbol? O carbon O sulfur O phosphorus O boron
A: Lewis symbol: Lewis symbol diagram is constructed by Putting dots around the element these dot…
Q: re around the central chlorine atom? neans one lone pair, one single bond, le bond. the arrangement…
A: As we know, Cl has 7 valence electrons. So, lewis structure of ClF3
Q: The value of Ka for hypochlorous acid (HClO) is 3.0 x 10-8 a. Write the chemical equation for the…
A: Here we are required to find the Gibbs free energy and standard Gibbs free energy for the…
Q: Balance the equation and calculate the E∘cell of the given reaction F2(g) + H2O(l) ------> 4F- (aq)…
A:
Q: Provide an etfficient synthesis of each molecule using the starting materials indicated. While no…
A: We have to carry out the required synthesis. The required transformation is shown below (a)
Q: 1. Gold-198 is used in the diagnosis of Ilver problems. If the half-life of Au-198 is 2.69 days: a…
A: Given-> Half life = 2.69 days Initial amount = 9.21 mg Time = 72.9 days
Q: Calculate the volume of 1.00M acetic acid and mass of sodium bicarbonate needed to make 50.0ml of a…
A:
Q: Consider the following reaction at 100.0 °C: Al(s) + NaOH(aq) + H20(l) ⇄ Na[Al(OH)4](aq) + H2(g) A.…
A: In this question, we have to calculate Kc for the given reaction and calculate Qc for the given…
Q: Draw a new purine base for DNA. Provide a name and a one-letter abbreviation for it.
A: The new purine base for DNA is Adenine. The one letter abbreviation for Adenine is A
Q: What would be the density (in g/L) of a sample of N2 gas at 70.0 °C and 2.50 atm of pressure?
A:
Q: 1. Give one example of chemical derived benzene. 2. Give the uses and source 3. Show the synthesis…
A: 1. Example for benzene derivatives are phenol, toluene and aniline. 2. Uses of Phenol - lower…
Q: Choose the proper IUPAC name of the following compound. p-fluorobenzoic acid O m-fluorobenzoic acid…
A:
Q: Calculate the pH of the solution that results when 0.093 g Mg(OH)2 is mixed with 75.0 mL of 0.0500 M…
A:
Q: Calculate the solubility at 25 °C of Zn (OH), in pure water and in a 0.0100M ZnSO, solution. You'lIl…
A: From alesk data, Ksp of Zn(OH)2=3.0×10-17 Molar mass of Zn(OH)2=99.424 g mol-1
Q: o bonds: a bonds:
A:
Q: decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not…
A: Entropy is degree of randomness or disorderness. By increasing Temperature, moles, volume Entropy…
Q: Determine the number of chiral carbon atoms in the follow- ing compound, and then calculate the…
A:
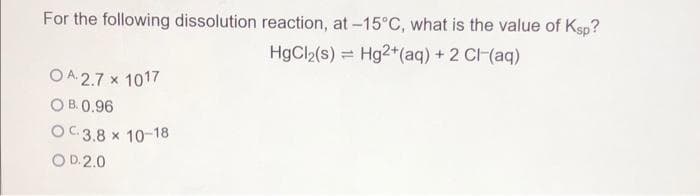
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Given the following data: PtCl42(aq)+2ePt(s)+4Cl(aq)Ered=0.73VPt2+(aq)+2ePt(s)Ered=1.20V Find Kffor PtCl42- at 25°C.For the following reaction: A(g) + 3 B(g) ⇌ C(g) + 2 D(g) Calculate the magnitude of Kp (don't report the units) assuming the following composition at equilibrium: PA = 0.61 bar PB = 0.926 bar PC = 0.24 bar PD = 0.779 barConsider the decomposition of a metal oxide to its elements, where M represents a generic metal. Substance ΔG°f(kJ/mol) M2O3(s) −8.00 M(s) 0 O2(g) 0 M2O3(s)↽−−⇀ 2M(s)+32O2(g)M2O3(s)↽−−⇀ 2M(s)+32O2(g) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? Δ?∘rxn=ΔGrxn°= kJ/molkJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? ?=K= What is the equilibrium pressure of O2(g) over M(s) at 298 K? ?O2=PO2= atm
- The solubility product constant (Ksp) for the following reaction is 5.61x10-9. What is the Free Energy of this reaction in kJ at 298 K? BaCO3(s) ↔ Ba2+(aq) + CO32-(aq)Using any data you can find in the ALEKS Data resource, calculate the quilibrium constant K at 25.0°C for the following reaction. Fe2O3(s)+ 3H2(g)→ 2Fe(s)+ 3H2O(l) Round your answer to 2 significant digits. delta G of Fe2O3=-742.2 kj/mol h2=0 Fe=0 H20=-237.1kj/molReaction KspKsp ΔH°ΔH° ΔS°ΔS° FeCO3(s)⇄Fe2+(aq)+CO32−(aq)FeCO3(s)⇄Fe2+(aq)+CO32−(aq) 3×10−113×10−11 <0<0 >0>0 MnCO3(s)⇄Mn2+(aq)+CO32−(aq)MnCO3(s)⇄Mn2+(aq)+CO32−(aq) 2×10−112×10−11 <0<0 >0 The table above lists the equilibrium constants and changes in thermodynamic properties for the dissolution of FeCO3 and MnCO3 at 25°C. The two-particle diagrams below represent saturated solutions of each compound at equilibrium. (see attached image) a.) The particle diagrams best represent that ΔH°<0ΔH°<0 because the ions from both compounds are solvated by water molecules. b.) The particle diagrams best represent that ΔH°<0ΔH°<0 because both compounds produce about the same amount of CO32−CO32− ions from the dissolution. c.) The particle diagrams best represent that ΔS°>0ΔS°>0 because both compounds produce a very small amount of ions from the dissolution. d.) The particle diagrams best represent that the molar solubility is greater for…
- The equilibrium constant for the reaction AgBr(s) Ag+(aq) + Br– (aq) is the solubility product constant, Ksp = 7.7 × 10–13 at 25°C. Calculate ΔG for the reaction when [Ag+] = 1.0 × 10–2 M and [Br–] = 1.0 × 10–3 M. Is the reaction spontaneous or nonspontaneous at these concentrations? (R = 8.314 J/K • mol) A. ΔG = –97.5 kJ/mol, nonspontaneous B. ΔG = 40.6 kJ/mol, nonspontaneous C. ΔG = 97.5 kJ/mol, spontaneous D. ΔG = 69.1 kJ/mol, nonspontaneous E. ΔG = –69.1 kJ/mol, spontaneous1. The solubility product constant for Ag3PO4 is 2.8 x10^-18. What is the solubility of silver ion in the solution? a. 5.37 x10 ^-5 b. 1.79 x10^-5 c. 2.8 x10^-15 d. 3.6 x10^-13 e. None of the above 2. Determine the equilibrium constant Kp at 25 C for the reaction: 2NO(g) + O2(g) ---> 2NO2 (g) delta G degree Rxn = -69.7 kJ/mol a. 1.65x10^12 b. 8.28 x10^-2 c. 2.6 d. 13.4 e. 6.07 x10^-13Determine the equilibrium constant for the system N2O4 2NO2 at 25°C. The concentrations are shown here: [N2O4] = 2.32 ´ 10-2 M, [NO2] = 1.41 ´ 10-2 M.
- The solubility product, Ksp, for the following solubility equilibrium at 25oC is 1.1 x 10-10. BaSO4(s)<--> Ba+2(aq) + SO4-2(aq) Calculate the standard free energy change for this equilibrium at this temperature. Group of answer choices 0.470 kJ/mol 56.8 kJ/mol 5.61 kJ/mol 4.77 kJ/molThe generic metal A forms in an insoluble salt Ab(s) and a complex AC5(aq) The equilibrium concentration in a solution of AC5 were found to be [A] = .100M, [C]=.0120M and [AC5]=.100M. Determine the formation canstant ,Kf of AC5 Kf=? The solubility of AB(s) in a 1.000M solution of C(aq) is found to be .143M What is the Ksp of AB? Ksp=?The equilibrium constant for the reaction: 2NO(g)+Br2(g)⇌2NOBr(g) is Kc=1.3×10−2 @ 1000 K. Calculate Kc for 2NOBr(g)⇌2NO(g)+Br2(g). Express your answer using two significant figures.



