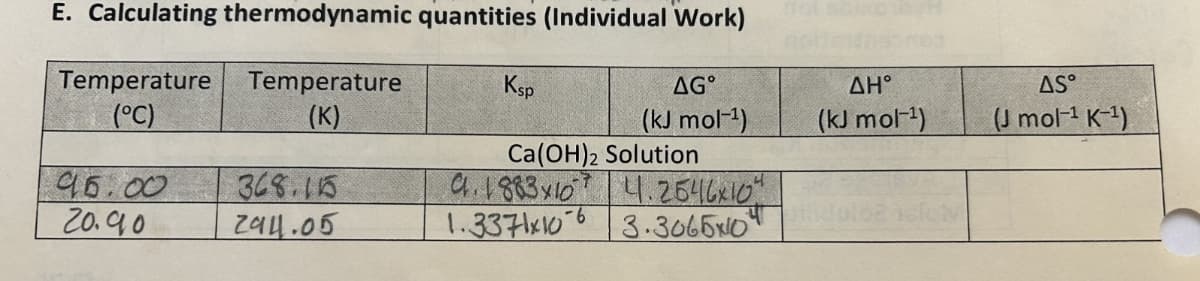
E. Calculating thermodynamic quantities (Individual Work) Temperature (°C) Temperature Ksp (K) AG° (kJ mol-1) 95.00 368.115 Ca(OH)2 Solution 9.1883x10 4.2646x10" 20.90 294.05 1.3371x106 3.30650 AH° (kJ mol-¹) AS (J mol¹ K-1)
Q: A 25.0 mL sample of 0.150 M NH3 is titrated with 0.180 M HCI. Kb of NH3 = 1.76 x 10-5 Show your…
A: Since you have posted a question with multiple sub-parts, we will provide the solution only for the…
Q: What is the approximate pH at the equivalence point of a weak base-strong acid titration if 50.00 mL…
A: Molarity of HCl = 0.1087 mol/LpKb = 4.75
Q: Draw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form…
A: The objective of the question is to draw the structure of the reactants.
Q: For each of the following cases, decide whether the pH is less than 7, equal to 7, or greater than…
A:
Q: We determined that the structure of BF3 is an exception to the octet rule (see above). 4. a)…
A: The formal charge on an atom in a molecule can be determined using the following equationThe valence…
Q: In the reaction below, which species is the electrophile? CI B H₂O OH AC B D H3O+ cr ဗ
A: Electrophiles are electron-deficient species that seek electrons to complete their octet or…
Q: Draw a structural formula for the product of the reaction shown. CH3 + CH3 • Use the wedge/hash bond…
A: The objective of the question is to predict the product formed in the given reaction.
Q: Balance the reaction below in acid by selecting the correct coefficient for each component.Cr2O72− +…
A: Cr2O7²⁻ + 3HNO₂ + 5H⁺ → 2Cr³⁺ + 3NO₃⁻ + 4H₂OExplanation: Here's how we achieved the balanced…
Q: Write structures for the carbonyl electrophile and enolate nucleophile that react to give the aldol…
A: Organic reactions are reactions in which organic reactants react with each other to produce organic…
Q: A certain weak base has a K₁ of 7.70 × 10-7. What concentration of this base will produce a pH of…
A: The objective of this question is to find the concentration of a weak base that will produce a…
Q: 2. Propose reagents for reducing the following carbonyl compounds: [7 x 2] 요? a) i Chemistry Steps…
A: Final answer is given in explanation please see from there.Explanation:Approach to solving the…
Q: 17. How many different products are possible for the following reaction? Consider both regio- and…
A: An arrow always depicts from a region of high electron density to low electron density ; that is…
Q: What element is represented by X in the atomic symbol notation 195 is over 78X ? Select one: A.…
A: The objective of the question is to identify the element represented by X in the atomic symbol…
Q: For the following reaction, 29.0 grams of calcium hydroxide are allowed to react with 23.7 grams of…
A:
Q: If you mix equimolar solutions of KCN and HCN O pH=pKa O pHfinal pHHCN O pHfinal pHHCN O pH=pKb O pH…
A: Given:Equimolar solutions of KCN and HCN are mixed.pH = pKapHfinal < pHHCNpHfinal > pHHCNpH =…
Q: Starting with the following reaction a more iron(II) chloride? Choose all th FeS(s) + 2 HCl(g) =…
A: Le Chatelier principle says that equilibrium will shift in such a way so as to undo the effect of…
Q: Draw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form…
A: The objective of the question is to predict the diene and dienophile for the given molecule.
Q: 10) Hydrocarbons are organic compounds consisting of hydrogen and carbon only. Burning hydrocarbons…
A: The objective of the question is to calculate the total pressure in the flask and the partial…
Q: The acid dissociation constant K of hydrocyanic acid (HCN) is 6.2 × 10 10 a Calculate the pH of a…
A:
Q: 2) [V(H2O)6]3+ (d2) complex have allowed electron transitions, as shown in the figure. Identify this…
A: The number and identification of electronic transitions in a complex can be done on the basis of…
Q: Draw the pyranose α-anomer chair conformation from the Fischer projection shown below. H OH H OH HO…
A: Anomers are stereoisomers of cyclic forms of carbohydrates that differ only in the configuration at…
Q: How much heat (in kJ) will be produced to cool off 50.0 g of Al from 81 0C to 180C ? (Specific heat…
A: Q = m × s × ∆TWhere, Q = heat evolved; m = mass of substance; s = specific heat and ∆T = change in…
Q: Calculate the pH of a 0.0269 M aqueous solution of the weak base piperidine (C5H1₁₁N, K₁ =…
A:
Q: The conducted experiment: In a 25 mL rbf add 5 mL of saturated sodium bicarbonate (NahCO3) , 0.5…
A: This reaction is known as Horner-Wadsworth-Emmons modification of Wittig reaction. Here a…
Q: please give a mechanism NH2
A: The objective of the question is to explain the reaction.
Q: Consider the titration of a 30.0 mL sample of 0.126 M CO32- so HI. Determine each quantity. Kb = 2.1…
A: The objective of this question is to determine the concentration of a specific reactant in a…
Q: B. Choose the major product for reaction I C. Choose the correct reagent for reaction IIA. HBrB.…
A: Final answer is given in explanation please see from there.Explanation:Approach to solving the…
Q: Question 6. Predict the product of the following reaction and draw the mechanism. Includes all…
A: Electrophilic substitution reaction of naphthaleneHalogens are ortho-para directing Carbocation…
Q: What is the pH at the equivalence point when 30. mL of 0.310 M hydroxylamine is titrated with 0.150…
A: The question is based on the concept of the pH of the solution.It is defined as a negative logarithm…
Q: Provide the major product for the following reaction? OH H
A: Aldehydes and ketones react with alcohol in acidic medium to form acetals and ketals. The reaction…
Q: A wastewater treatment plant (WWTP) releases effluent into a stream with mean depth 2 m and mean…
A: Please see the attached image for the answer.Explanation:C.In the context of river pollution…
Q: A solution is made initially with 0.200 M HIO3 (Kc = 0.170). Once the equilibrium below is…
A: For the given reaction,Equilibrium constant,The concentration of The equilibrium concentration of…
Q: Predict the relative rates of these reactions. That is, select 1 next to the reaction with the…
A: Factors affecting the reaction rates :Nature of substrate:a. Primary alkyl halide: SN2 substitution,…
Q: IVe the name and formula for the following coordination compound: 21 CI NH2111 NH3 Cr NH3 N H₂ NH3 E
A: Here we need to find the name of the coordination compound and its formula.In writing the name…
Q: 4.00 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have…
A:
Q: For the following molecule, enter the number of carbon and hydrogen atoms in the spaces provided.…
A: In a bond line structure, carbon -carbon bonds are represented using lines.The intersection point of…
Q: Balance the following redox reaction in basic solution using the half reactions method. Zn (s) +…
A: The objective of this question is to balance the given redox reaction in basic solution using the…
Q: Rate constant k for a first order reaction has B ound to be 2.54 × 10-3 sec¯¹. Calculate its 3/4th…
A:
Q: Consider the reaction of 67.1 mL of 0.310 M NaC, H₂O₂ with 50.0 mL of 0.245 M HBr. (Ka of HC,HO₂ =…
A: When HBr reacts with NaC7H5O2, NaBr and HC7H5O2 are formed.Now, the Ka value of HC7H5O2 is..So,…
Q: Suppose a 250. mL flask is filled with 0.70 mol of H2 and 1.1 mol of I2. The following reaction…
A: Find the initial concentration of hydrogen and iodide.Molarity = moles /volume Molarity of H2=…
Q: 1. Determine the product(s) and propose a mechanism for the following reaction. OH MeOH H+ lottex (1
A: The objective of this question is to determine the product(s) and propose a mechanism for the given…
Q: You have an aliquot of 11 mL of 0.1093 mol/L phosphoric acid. The ka values for phosphoric acid are…
A: Volume of phosphoric acid = 11 mLMolarity of phosphoric acid sample = 0.1093 mol/LVolume of NaOH…
Q: Predict the major product of the following reaction: CCH-CH₂CCI AICI; о о об
A: Friedel crafts acylation reaction: Benzene reacts with acyl chloride in presence of AlCl3 to form…
Q: Assign the following stereocenter as having the R or S configuration. H- Br Br -H Give detailed…
A: To solve this problem we have to give the configuration of stereocenter of the given compound. To…
Q: 9.46 Draw the substitution product formed (including stereochemistry) when (R)-hexan-2-ol is treated…
A: The objective of the question is to determine the substitution products formed when (R)-hexan-2-ol…
Q: Given the following unbalanced reaction: Ti+HCI TiCl3 + H2 If the percent yield is 86.8%, what mass…
A: The balanced equation in this case will be:
Q: Br я ONa DMF aprotic 2° SN or SN2?
A: An arrow always depicts from a region of high electron density to low electron density ; that is…
Q: Considering the reaction below, what is the rate equation of it? Br B NaCN Acetone CN D: Br: Rate =…
A: Answer:The law of mass action states that rate of reaction is directly proportional to all the…
Q: Draw the complete mechanism for each of the following polar reactions. a.1 H-Br Br Write a mechanism…
A: Alkene reacts with Hydrogen halide to form alkyl halide through a carbocation intermediate.The first…
Q: What [Ag+] (in M) is required to reduce the [CrO42] in a solution to 8.1× 10-4 M by precipitation of…
A: The objective of the question is to find the concentration of Ag+ ions required to reduce the…
Step by step
Solved in 5 steps with 13 images

- The enthalpy of vaporization of liquid diethyl ether, (C2H5)2O, is 26.0 kJ/mol at the boiling point of 35.0 °C. Calculate ΔS° for a vapor-to-liquid transformation at 35.0 °C.A 1 mol quantity of hydrogen gas sample was heated at constant pressure from a temperature of 300 K to 500 K. With the entropy transformation process as a function of temperature variation, the heat capacity equation at constant pressure was estimated , Cp = 6.9469 - 0.199 x 10-3 T + 4.808 x 10-7 T2 (J K-1mol-1). Determine the entropy change of the process.Please help answer unanswered parts: Calculations 1 and 2 (at bottom, red boxes) Equation used was as follows: C [crystal graphite] + CO2 [gas ] ⇌ 2CO [gas ] ; Formation of carbon monoxide Equation: C [crystal graphite] + CO2 [gas] + <---> 2CO [gas] + + Standard Enthalpy of Formation and Entropy ∆fHo(T) (KJ/mol) So(T) (J/mol.K) C [crystal graphite] 18.51 30.32 CO2 [gas] -343.41 283.87 CO [gas] -78.69 243.42
- The temperature of the steam coming from the steam boiler to the steam engine cylinder is 120 °C. Steam condensed in a cold reservoir at 40 °C. What is the maximum work. What is done by the engine under ideal conditions with 4.2 kJ of heat absorbed?What is the temperature of the high-temperature reservoir of a process that has an efficiency of 44.0% (0.440) and a low-temperature reservoir at 150°C?The standard Gibbs energy of formation of rhombic sulfur is zero, and that of monoclinic sulfur is +0.33 kJ mol−1 at 25 °C. The standard molar entropy of rhombic sulfur is 31.80 J K−1 mol−1, and that of monoclinic sulfur is 32.6 J K−1 mol−1. At what temperature will the transition occur at 1 bar? _______ K. 3 sig. fig.
- The vaporisation of a certain element at 25.0 oC has the following enthalpy and entropy values: Hvap = 1.00 kJ mol-1 and Svap = 156 J K-1 mol-1. What is the total entropy change (Stot) for the vaporisation of this element, in J K-1mol-1?Consider the following reaction: H2(g) + ½ O2 (g) ------> H2O (g) The standard enthalpy of formation of gaseous H2O at 298 K is -241.82 kJ mol-1. Calculate the value at 153 0C. Given Cp,m for H2O(g): 33.58 kJ mol-1; H2 (g): 28.84 kJ mol-1; O2 (g): 29.37 kJ mol-1. Assume heat capacities are independent of T. NOTE:answer in kilojoules per mole (kJ/mol)The vaporisation of a certain element at 21 degrees Celsius had the following enthalpy and entropy values: ∆Hvap= 3.40 KJ mol-1 and ∆Svap= 246 J K-1mol-1 What is the total entropy change (∆Stot) for the vaporisation of this element in J K-1 mol-1? With correct significant figures.
- When nitric acid is produced industrially, nitrogen monoxide, NO, is first formed at high temperature. Bakefetr reacts NO on cooling further with oxygen to nitrogen dioxide: 2 NO(g) + O2 ⇌ 2 NO2 (g) Table 1: Thermodynamic data at 25°C. Bond ΔfHom Som Cop,m NO(g) 90.25 210.76 29.34 O2(g) 0.00 205.14 29.36 NO2(g) 33.18 240.06 37.20 1) Calculate (with all relevant intermediate calculations) the standard reaction Gibbs free energy, ΔrG25o, for reaction (1) at 25°C from the data in Table 1 2) Calculate (with all relevant intermediate calculations) the equilibrium constant K25, for reaction (1) at 25°C. 3) Industrially, however, the reaction does not proceed at 25°C but at 500°C. Therefore, calculate (with all relevant intermediate calculations) the standard reaction Gibbs free energy, ΔrG500o, for reaction (1) at 500°C under the assumption that the standard molar heat capacities, Cop, in Table 1 are independent of temperature in the interval [25°C, 500°C]A. Say 0.0101 moles of gas in a car engine cylinder under a pressure of 608 kPa at 882 K expands adiabatically and irreversibly against 1 atm pressure. The Cv,m of the gas is 20.8 J/K/mol while Cp,m = 29.12 J/K/mol, so what is q, Vi, Tf, Pf, U, w, and H? B. Say 0.0101 moles of gas in a car engine cylinder at atmospheric pressure at 90 °C contractsadiabatically and reversibly until it is at 608 kPa pressure. The Cv,m of the gas is 20.8 J/K/molwhile Cp,m = 29.12 J/K/mol, so what is q, Tf, U, w, and H? Hint: (Pi/Pf)= (Ti/Tf)^(CV+nR/nR)What does the superscript ° indicate when associated with a thermodynamicquantity, as in ∆H °, ∆S°, or ∆G°?

