Each row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type (check all that apply) solution initial components effect of change on pH (check one) change O acidic O pH higher H,0, H Br O basic add KOH O pH lower O neutral pH the same O acidic pH higher H,0 add Na OH O PH lower basic O neutral pH the same O acidic pH higher H,0 add NaI O pH lower basic O neutral O pH the same acidic pH higher H,0, HBr O basic add K Br pH lower D pH the same neutral Fynlanation Check 2021 McGraw-Hill Educa O O0 0 000
Each row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type (check all that apply) solution initial components effect of change on pH (check one) change O acidic O pH higher H,0, H Br O basic add KOH O pH lower O neutral pH the same O acidic pH higher H,0 add Na OH O PH lower basic O neutral pH the same O acidic pH higher H,0 add NaI O pH lower basic O neutral O pH the same acidic pH higher H,0, HBr O basic add K Br pH lower D pH the same neutral Fynlanation Check 2021 McGraw-Hill Educa O O0 0 000
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter13: Acids And Bases
Section: Chapter Questions
Problem 98QAP: Consider the following six beakers. All have 100 mL of aqueous 0.1 M solutions of the following...
Related questions
Question
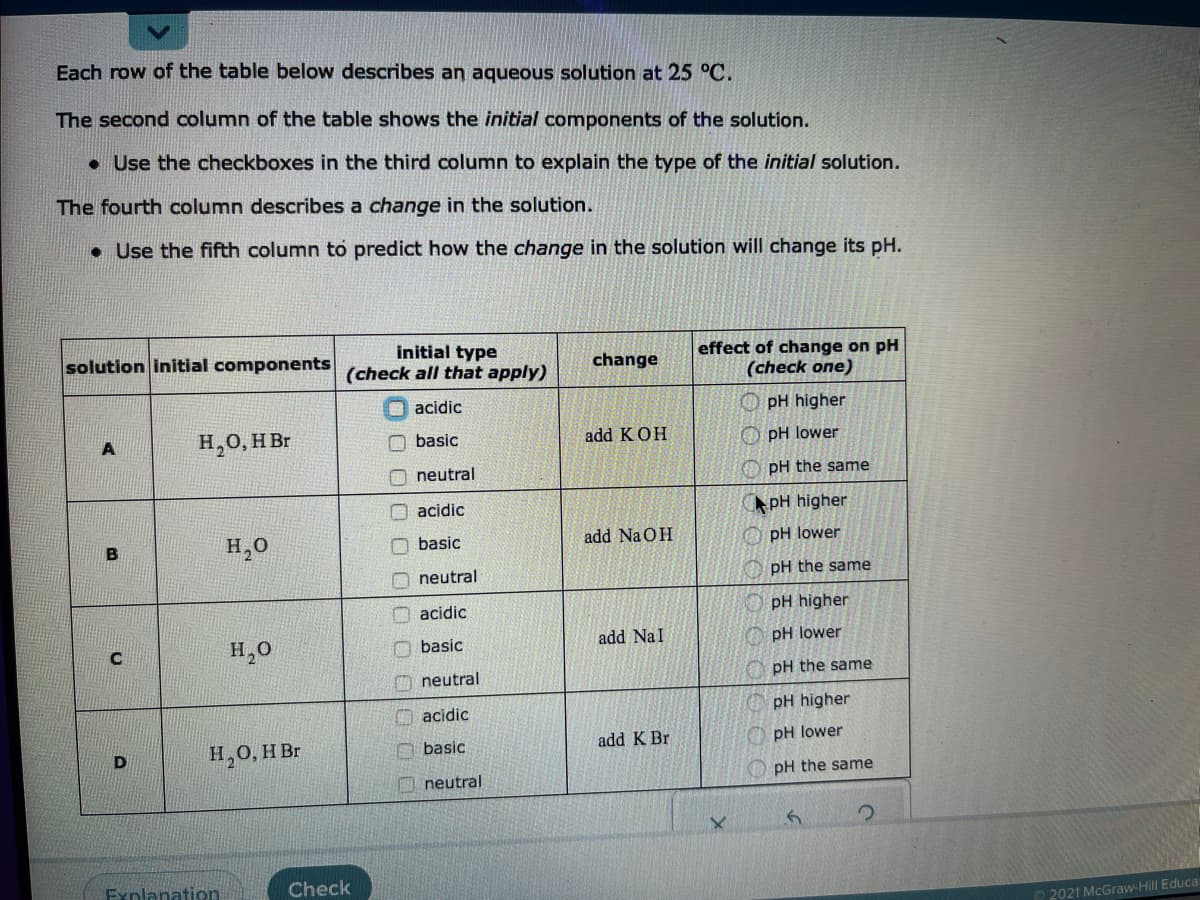
Transcribed Image Text:Each row of the table below describes an aqueous solution at 25 °C.
The second column of the table shows the initial components of the solution.
• Use the checkboxes in the third column to explain the type of the initial solution.
The fourth column describes a change in the solution.
• Use the fifth column to predict how the change in the solution will change its pH.
initial type
(check all that apply)
solution initial components
effect of change on pH
(check one)
change
O acidic
O pH higher
H,0, H Br
O basic
add KOH
O pH lower
neutral
pH the same
O acidic
pH higher
H,0
O basic
add Na OH
pH lower
neutral
pH the same
O acidic
pH higher
H,0
O basic
add NaI
O pH lower
O neutral
pH the same
acidic
pH higher
H,0, H Br
O basic
add K Br
pH lower
pH the same
O neutral
Exnlanation
Check
2021 McGraw-Hill Educa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co
