Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.20QAP
Related questions
Question
Each solution began with a different [Ag+]0. Should the Ksp value be the same for each solution? Explain?
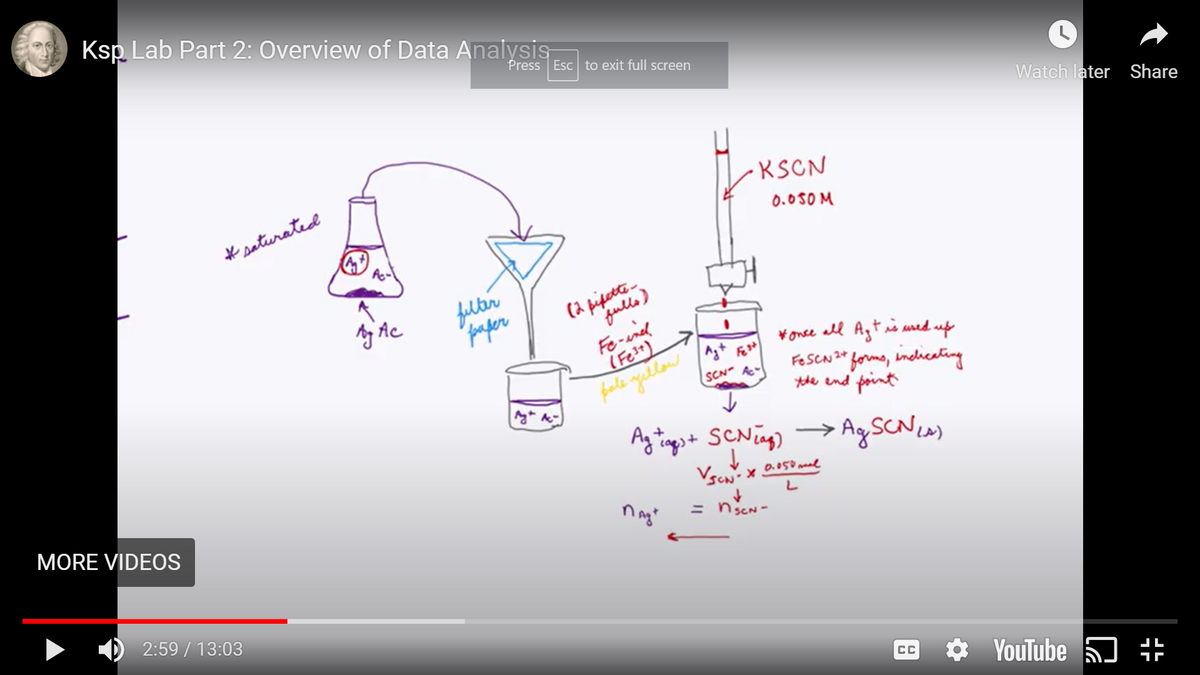
Transcribed Image Text:Ksp Lab Part 2: Overview of Data Analysis
Press Esc to exit full screen
Watch later Share
KSCN
# saturated
0.0SOM
filter
paper
(2 pigette
fulls)
A Ac
Fe-ind
(Fe?") >
¥once all Agtis ured up
FeseNze forme, indicating
the end point
SCN Ac-
Ag SCNIA)
MORE VIDEOS
ngt
= nscN-
2:59 / 13:03
O YouTube 5.
CC
+
![A Determination of Ksp Lab: X
b Similar Questions | bartlet X
Grammarly
M Inbox (1) - cmc640@scarl X
G Each solution began with × +
A camdencc.instructure.com/courses/18740/pages/determination-of-ksp-lab?module_item_id=790778
C
T. Idbie -
COmpiete ICE (apIE OI 5OIULIOIT C T CAiculdLIOIT OI I1 sn
II. Error
A. Figure 4 - Calculate the percent error of the Ksp for each solution. The solubility of A9C2 H3O2 at room temp
1.20g
is
100.mL
(hint: review how to calculate Ksp from solubility data).
B. Discuss one or two experimental errors that were made that could have contributed to the percent errors calcu
V. Conclusion
A. Recap the purpose/goal of the lab (according to the rubric)
B. Each solution began with a different [Ag+]o. Should the Ksp value be the same for each solution? Explain?
« Previous
55°F
3:01 PM
Mostly cloudy
4/27/2022
>](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F71d7a67c-bd18-4e8c-9024-55cc75fdbac3%2F80a0e379-cd71-42ad-81c9-28b206073998%2F28zl5eb_processed.png&w=3840&q=75)
Transcribed Image Text:A Determination of Ksp Lab: X
b Similar Questions | bartlet X
Grammarly
M Inbox (1) - cmc640@scarl X
G Each solution began with × +
A camdencc.instructure.com/courses/18740/pages/determination-of-ksp-lab?module_item_id=790778
C
T. Idbie -
COmpiete ICE (apIE OI 5OIULIOIT C T CAiculdLIOIT OI I1 sn
II. Error
A. Figure 4 - Calculate the percent error of the Ksp for each solution. The solubility of A9C2 H3O2 at room temp
1.20g
is
100.mL
(hint: review how to calculate Ksp from solubility data).
B. Discuss one or two experimental errors that were made that could have contributed to the percent errors calcu
V. Conclusion
A. Recap the purpose/goal of the lab (according to the rubric)
B. Each solution began with a different [Ag+]o. Should the Ksp value be the same for each solution? Explain?
« Previous
55°F
3:01 PM
Mostly cloudy
4/27/2022
>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
