earning Goal: o understand several methods of measuring gas ressure. Part A iases exert a measurable pressure (P) on the alls of their container. The Sl unit for pressure is he pascal (Pa). Other pressure units frequently sed in chemistry are millimeters of mercury mmHg) and atmospheres (atm). You can onvert between units of pressure using conversion actors such as those indicated below. atm = 760 mmHg = 101, 325 Pa On a rainy day, a barometer reads 727 mmHg . Convert this value to atmospheres. Express your answer numerically in atmospheres. • View Available Hint(s) ΑΣφ ? atm gure < 1 of 1 > Part B A closed container is filled with oxygen. The pressure in the container is 405 kPa . What is the pressure in millimeters of mercury? Express the pressure numerically in millimeters of mercury. Gas • View Available Hint(s) 18 cm | ΑΣφ mmHg
earning Goal: o understand several methods of measuring gas ressure. Part A iases exert a measurable pressure (P) on the alls of their container. The Sl unit for pressure is he pascal (Pa). Other pressure units frequently sed in chemistry are millimeters of mercury mmHg) and atmospheres (atm). You can onvert between units of pressure using conversion actors such as those indicated below. atm = 760 mmHg = 101, 325 Pa On a rainy day, a barometer reads 727 mmHg . Convert this value to atmospheres. Express your answer numerically in atmospheres. • View Available Hint(s) ΑΣφ ? atm gure < 1 of 1 > Part B A closed container is filled with oxygen. The pressure in the container is 405 kPa . What is the pressure in millimeters of mercury? Express the pressure numerically in millimeters of mercury. Gas • View Available Hint(s) 18 cm | ΑΣφ mmHg
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.55P
Related questions
Question
Please answer question 1 Part A, B, and C
Transcribed Image Text:Learning Goal:
To understand several methods of measuring gas
Part A
pressure.
Gases exert a measurable pressure (P) on the
walls of their container. The Sl unit for pressure is
the pascal (Pa). Other pressure units frequently
used in chemistry are millimeters of mercury
(mmHg) and atmospheres (atm). You can
convert between units of pressure using conversion
factors such as those indicated below.
On a rainy day, a barometer reads 727 mmHg . Convert this value to atmospheres.
Express your answer numerically in atmospheres.
View Available Hint(s)
1 atm = 760 mmHg = 101, 325 Pa
?
atm
Figure
1 of 1
Part B
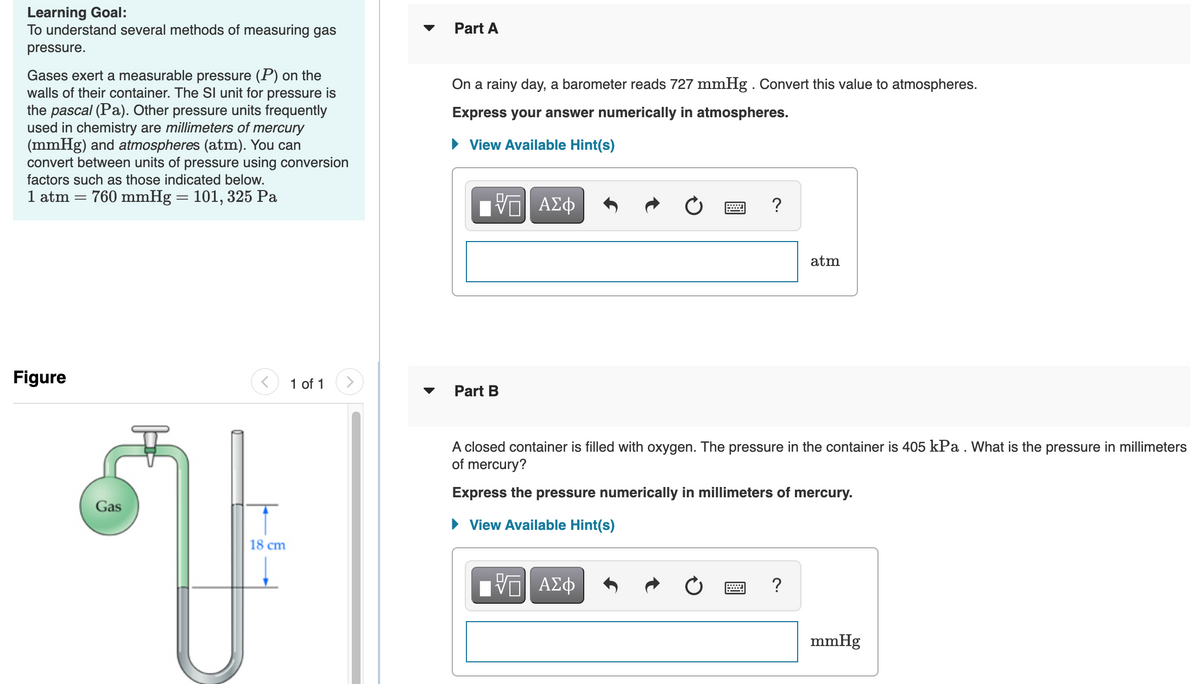
A closed container is filled with oxygen. The pressure in the container is 405 kPa . What is the pressure in millimeters
of mercury?
Express the pressure numerically in millimeters of mercury.
Gas
• View Available Hint(s)
18 cm
Ηνα ΑΣφ
?
mmHg
Transcribed Image Text:Part C
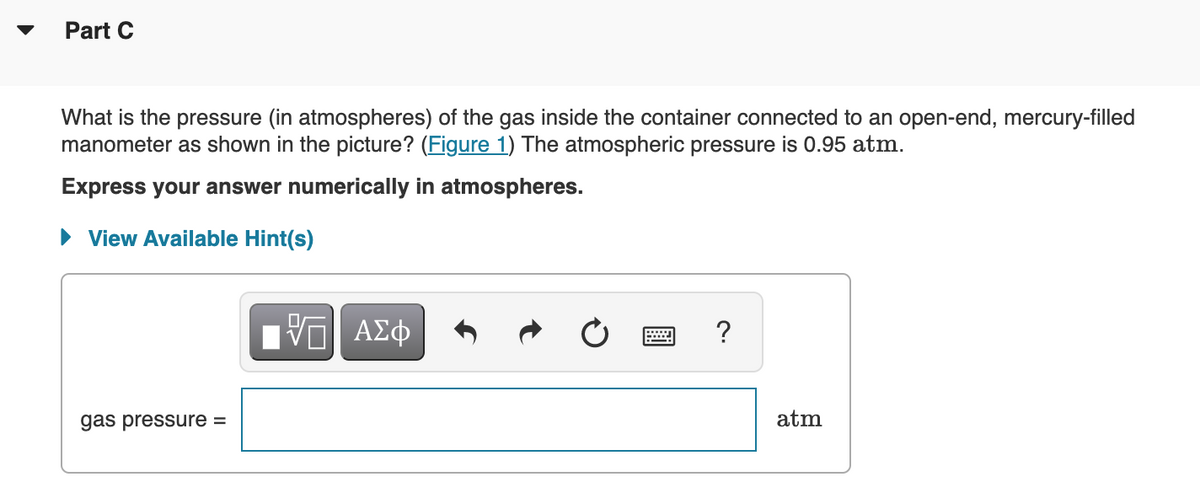
What is the pressure (in atmospheres) of the gas inside the container connected to an open-end, mercury-filled
manometer as shown in the picture? (Figure 1) The atmospheric pressure is 0.95 atm.
Express your answer numerically in atmospheres.
• View Available Hint(s)
ΑΣφ
?
gas pressure =
atm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
