In the fermentation of glucose (wine making), 740 mL of CO2 gas was produced at 38 °Č and 1.15 atm Part A You may want to reference (Pages 272 - 273) Section 8.5 while completing this problem. What is the final volume, in liters, of the gas when measured at 25 °C and 695 mmHg, when n is constant? Express the volume in liters to two significant figures. ? Vco, = L Previous Answers Request Answer Submit
In the fermentation of glucose (wine making), 740 mL of CO2 gas was produced at 38 °Č and 1.15 atm Part A You may want to reference (Pages 272 - 273) Section 8.5 while completing this problem. What is the final volume, in liters, of the gas when measured at 25 °C and 695 mmHg, when n is constant? Express the volume in liters to two significant figures. ? Vco, = L Previous Answers Request Answer Submit
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.93PAE: 93 The complete combustion of octane can be used as a model for the burning of gasoline:...
Related questions
Question
Transcribed Image Text:<Chapters 5, 8, 9 ,10 in a nutshell
Problem 8.84 - Enhanced - with Feedback
19 of 50
>
I Review | Constants | Periodic Table
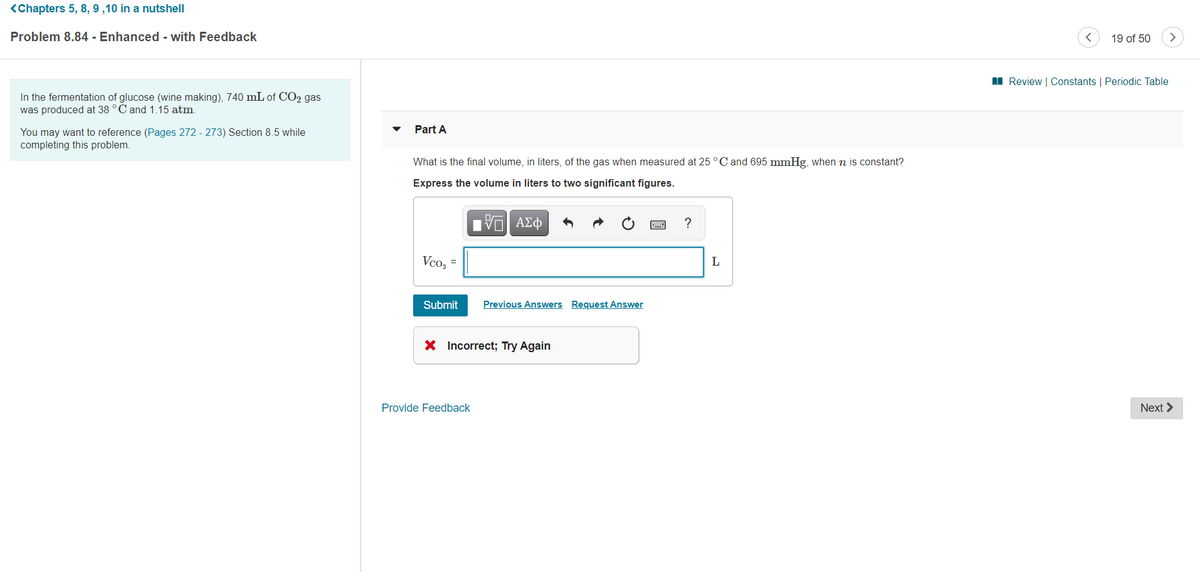
In the fermentation of glucose (wine making), 740 mL of CO2 gas
was produced at 38 °C and 1.15 atm.
Part A
You may want to reference (Pages 272 - 273) Section 8.5 while
completing this problem.
What is the final volume, in liters, of the gas when measured at 25 °C and 695 mmHg, when n is constant?
Express the volume in liters to two significant figures.
Vco, =
L
Submit
Previous Answers Request Answer
X Incorrect; Try Again
Provide Feedback
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning