ELECTRON DOMAIN BONDING LONE PAIR MOLECULAR GEOMETRY EXAMPLE 90 6. octahedral SF6 square pyramidal 1 BRF5 square planar XEF4 1)Refer the above diagram.The molecular geometry of SF6 is octahedral.Draw the structure.Do it step by step and explain.be very clear and accurate. 2)Refer the above diagram.The molecular geometry of BrF5 is square pyramidal Draw the structure.Do it step by step and explain.be very clear and accurate. 3)Refer the above diagram.The molecular geometry of XeF4 is square planar Draw the structure.Do it step by step and explain.be very clear and accurate. 2) 4)
ELECTRON DOMAIN BONDING LONE PAIR MOLECULAR GEOMETRY EXAMPLE 90 6. octahedral SF6 square pyramidal 1 BRF5 square planar XEF4 1)Refer the above diagram.The molecular geometry of SF6 is octahedral.Draw the structure.Do it step by step and explain.be very clear and accurate. 2)Refer the above diagram.The molecular geometry of BrF5 is square pyramidal Draw the structure.Do it step by step and explain.be very clear and accurate. 3)Refer the above diagram.The molecular geometry of XeF4 is square planar Draw the structure.Do it step by step and explain.be very clear and accurate. 2) 4)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter5: Alkenes: Bonding, Nomenclature, And Properties
Section: Chapter Questions
Problem 5.11P: The structure of 1,2-propadiene (allene) is shown to the right. (a) Predict all approximate bond...
Related questions
Question
! ( plz do all with detail explanation , if you have plan to do only 1 plz skip lets others do all , these are very easy , give ans with explanation )
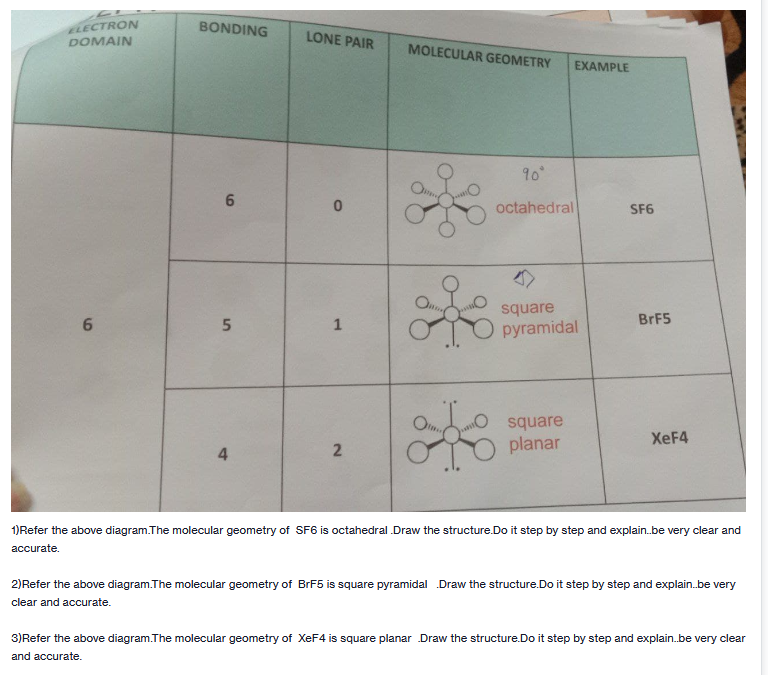
Transcribed Image Text:ELECTRON
DOMAIN
BONDING
LONE PAIR
MOLECULAR GEOMETRY
EXAMPLE
90
octahedral
SF6
square
pyramidal
BRF5
square
XEF4
planar
4
1)Refer the above diagram.The molecular geometry of SF6 is octahedral Draw the structure.Do it step by step and explain.be very clear and
accurate.
2)Refer the above diagram.The molecular geometry of BrF5 is square pyramidal Draw the structure.Do it step by step and explain.be very
clear and accurate.
3)Refer the above diagram.The molecular geometry of XEF4 is square planar Draw the structure.Do it step by step and explain.be very clear
and accurate.
1.
2.
5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning