Give the approximate bond angle for a molecule with an octahedral shape. 180° 105° 90° 109.5° 120° QUESTION 41 Determine the electron geometry (eg) and molecular geometry (mg) of NCI 3. eg = tetrahedral, mg = trigonal pyramidal %3D %3D eg = tetrahedral, mg = tetrahedral eg = trigonal planar, mg = bent eg = linear, mg = linear eg = linear, mg = trigonal planar %3D
Give the approximate bond angle for a molecule with an octahedral shape. 180° 105° 90° 109.5° 120° QUESTION 41 Determine the electron geometry (eg) and molecular geometry (mg) of NCI 3. eg = tetrahedral, mg = trigonal pyramidal %3D %3D eg = tetrahedral, mg = tetrahedral eg = trigonal planar, mg = bent eg = linear, mg = linear eg = linear, mg = trigonal planar %3D
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter5: Chemical Bonding: The Covalent Bond Model
Section: Chapter Questions
Problem 5.81EP: Indicate whether each of the following molecules is polar or nonpolar. The molecular geometry is...
Related questions
Question
100%
40. 41.
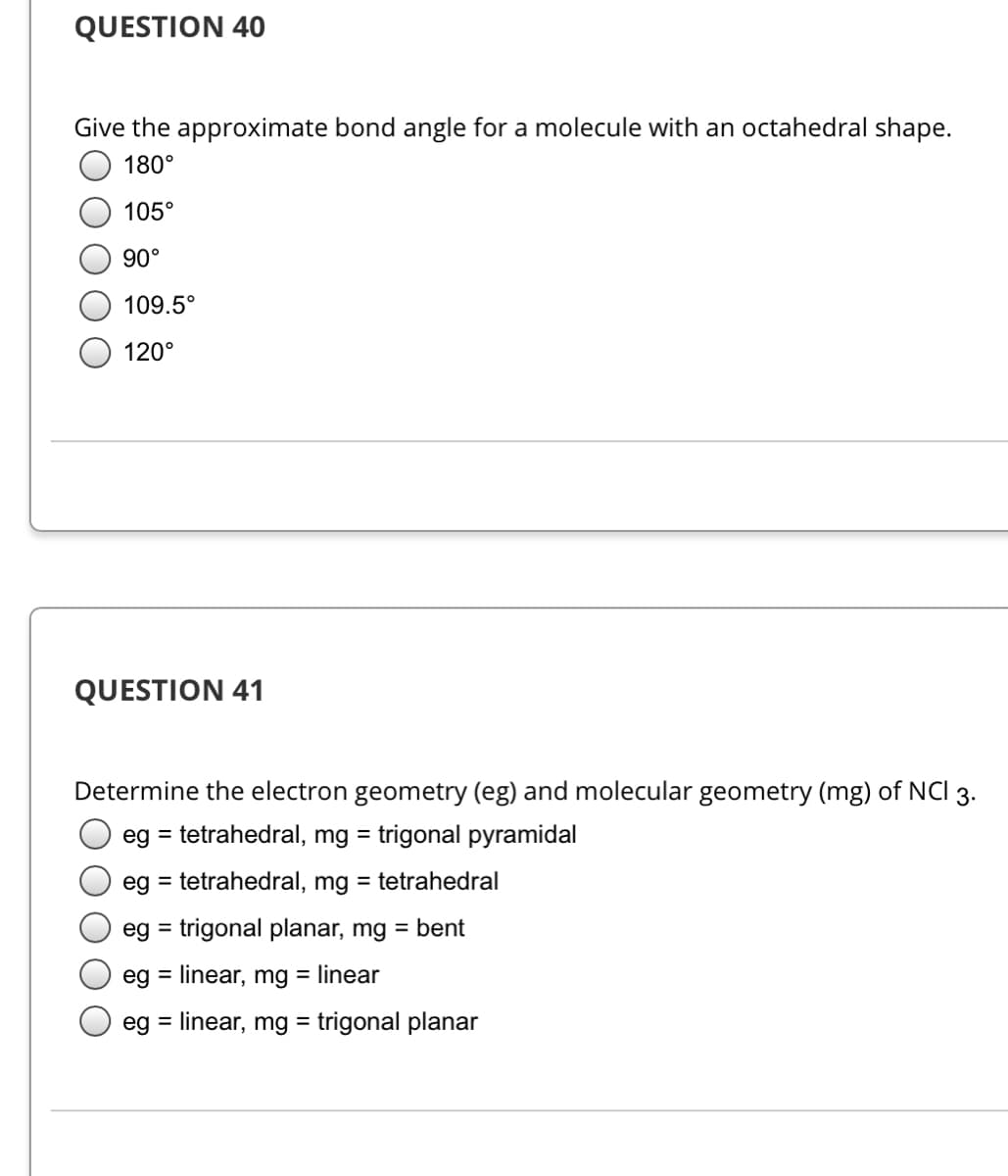
Transcribed Image Text:QUESTION 40
Give the approximate bond angle for a molecule with an octahedral shape.
180°
105°
90°
109.5°
120°
QUESTION 41
Determine the electron geometry (eg) and molecular geometry (mg) of NCI 3.
eg = tetrahedral, mg = trigonal pyramidal
eg = tetrahedral, mg = tetrahedral
eg = trigonal planar, mg = bent
%3D
eg = linear, mg = linear
eg = linear, mg = trigonal planar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning