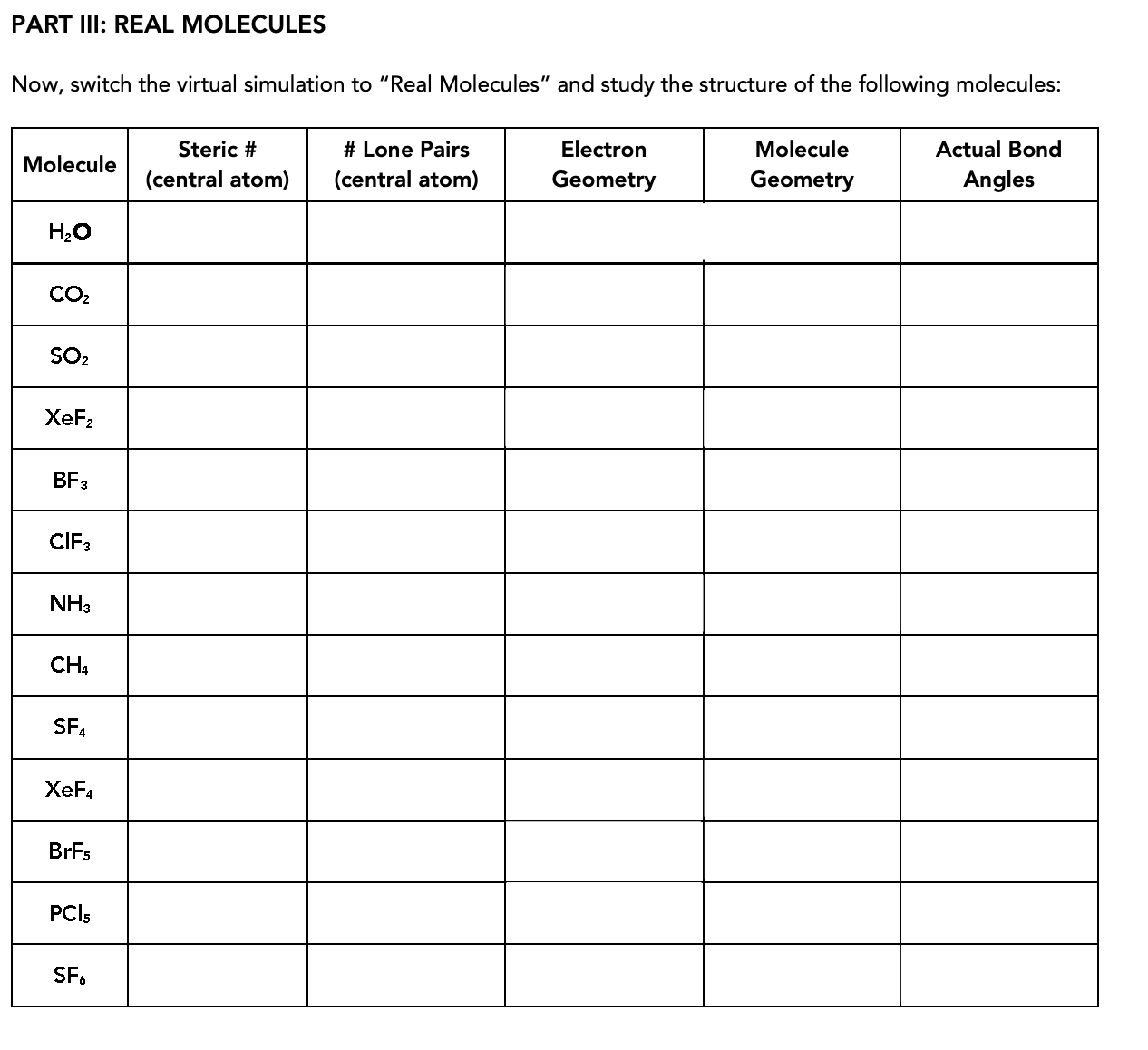
PART III: REAL MOLECULES Now, switch the virtual simulation to "Real Molecules" and study the structure of the following molecules: Steric # # Lone Pairs Electron Molecule Actual Bond Molecule (central atom) (central atom) Geometry Geometry Angles Н.о CO2 SO2 XeF2 BF3 CIF3 NH3 CH4 SF4 XeF4 BRFS PCI5 SF. 8. In the trigonal bipyramidal model, there are two sets of bond angles (90°/180° and 120°). Equatorial atoms are separated by the 120° angles and the axial ones involve the 90°/180° angles. In the diagram, which atoms could be identified as equatorial and which as axial? B C. A.
PART III: REAL MOLECULES Now, switch the virtual simulation to "Real Molecules" and study the structure of the following molecules: Steric # # Lone Pairs Electron Molecule Actual Bond Molecule (central atom) (central atom) Geometry Geometry Angles Н.о CO2 SO2 XeF2 BF3 CIF3 NH3 CH4 SF4 XeF4 BRFS PCI5 SF. 8. In the trigonal bipyramidal model, there are two sets of bond angles (90°/180° and 120°). Equatorial atoms are separated by the 120° angles and the axial ones involve the 90°/180° angles. In the diagram, which atoms could be identified as equatorial and which as axial? B C. A.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter8: Bonding: General Concepts
Section: Chapter Questions
Problem 122E: Predict the molecular structure (including bond angles) for each of the following. (See Exercises...
Related questions
Question
Transcribed Image Text:PART III: REAL MOLECULES
Now, switch the virtual simulation to "Real Molecules" and study the structure of the following molecules:
Steric #
# Lone Pairs
Electron
Molecule
Actual Bond
Molecule
(central atom)
(central atom)
Geometry
Geometry
Angles
Н.о
CO2
SO2
XeF2
BF3
CIF3
NH3
CH4
SF4
XeF4
BRFS
PCI5
SF.
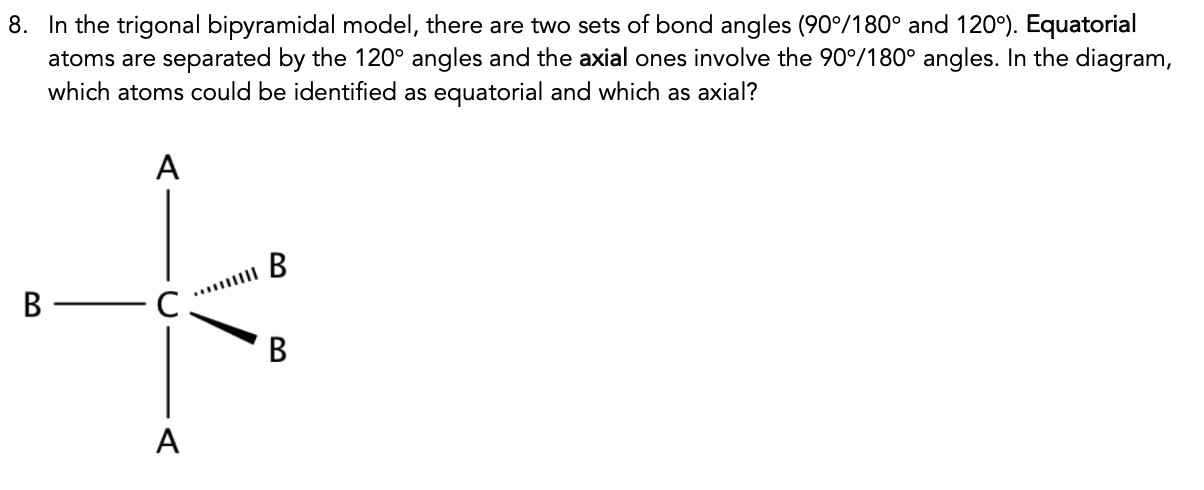
Transcribed Image Text:8. In the trigonal bipyramidal model, there are two sets of bond angles (90°/180° and 120°). Equatorial
atoms are separated by the 120° angles and the axial ones involve the 90°/180° angles. In the diagram,
which atoms could be identified as equatorial and which as axial?
B
C.
A.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning