Electronegativity and Polarity (Here's a link to an Electronegativity Chart) 8. What is electronegativity, and might differences in electronegativity help us determine if a pond is polar or nonpolar (or ionic)?
Electronegativity and Polarity (Here's a link to an Electronegativity Chart) 8. What is electronegativity, and might differences in electronegativity help us determine if a pond is polar or nonpolar (or ionic)?
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter12: Chirality
Section: Chapter Questions
Problem 9E
Related questions
Question
Please answer for question 17 and 18
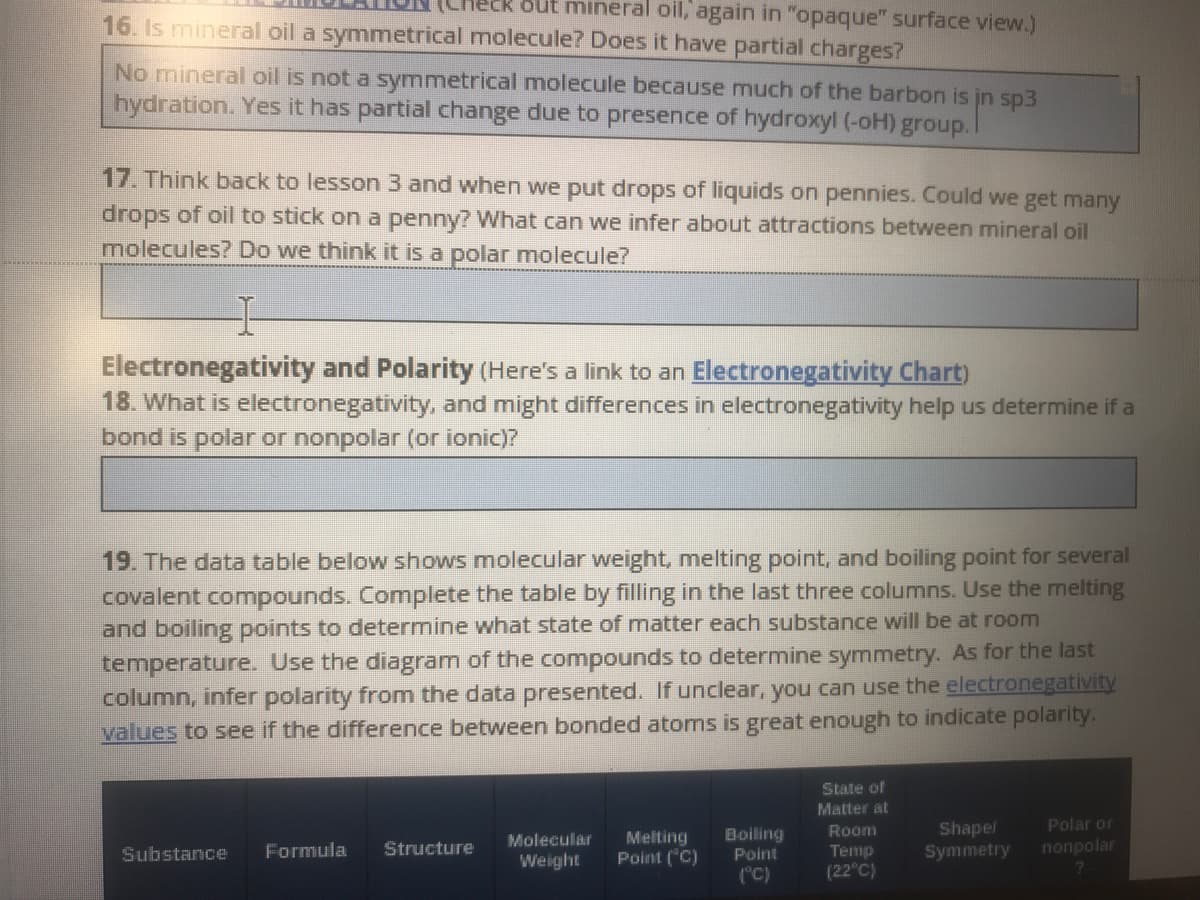
Transcribed Image Text:out mineral oil, again in "opaque" surface view.)
16. Is mineral oil a symmetrical molecule? Does it have partial charges?
No mineral oil is not a symmetrical molecule because much of the barbon is jn sp3
hydration. Yes it has partial change due to presence of hydroxyl (-oH) group.I
17. Think back to lesson 3 and when we put drops of liquids on pennies. Could we get many
drops of oil to stick on a penny? What can we infer about attractions between mineral oil
molecules? Do we think it is a polar molecule?
Electronegativity and Polarity (Here's a link to an Electronegativity Chart)
18. What is electronegativity, and might differences in electronegativity help us determine if a
bond is polar or nonpolar (or ionic)?
19. The data table below shows moleccular weight, melting point, and boiling point for several
covalent compounds. Complete the table by filling in the last three columns. Use the melting
and boiling points to determine what state of matter each substance will be at room
temperature. Use the diagram of the compounds to determine symmetry. As for the last
column, infer polarity from the data presented. If unclear, you can use the electronegativity
values to see if the difference between bonded atoms is great enough to indicate polarity.
State of
Matter at
Room
Shapel
Polar or
Melting
Point ("C)
Boiling
Point
Molecular
Formula
Structure
nonpolar
Теmp
(22°C)
Symmetry
Substance
Weight
("C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER