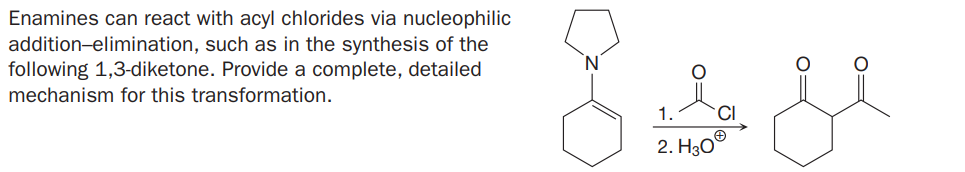
Enamines can react with acyl chlorides via nucleophilic addition-elimination, such as in the synthesis of the following 1,3-diketone. Provide a complete, detailed mechanism for this transformation. 1. 2. Hао
Q: 5. Provide a stepwise synthesis for the following.
A: The stepwise synthesis is shown below
Q: `NH HNO3 `NH H2SO4 NO2 Acetanilide para-Nitroacetanilide
A: We have to draw the curly arrow mechanism for this reaction showing step by step and also draw two…
Q: provide a possible synthesis for the following reaction
A:
Q: Provide a plausible synthetic pathway for the following organic transformation starting with acetic…
A:
Q: Tamoxifen is an estrogen receptor modulator that is used in the treatment of breast cancer. Provide…
A: In organic synthesis, suitable reagent converts the reactants into products. Reagents attacks with…
Q: Propose a reasonable synthetic route for the following conversion. Show all isolated intermediates.
A: Answer is given in below image
Q: Provide a detailed curved arrow mechanism for the following transformation H300 C Hz
A: The mechanism is drawn in the following steps.
Q: (−)-Hyoscyamine, an optically active drug used to treat gastrointestinal disorders, is isolated from…
A: (a) (-)-Hyoscyamine contains two hydrogens which are acidic, which are benzylic and alcoholic…
Q: Of the following, which represents a logical retrosynthesis for target molecule A? to OH MgBr MgBr…
A: Given Molecule
Q: Terreic acid, shown below, is a naturally occurring antibiotic metabolite of the fungus Aspergillus…
A: Given molecule:
Q: OH
A: Applying concept of organic synthesis.
Q: please help with this question. thank you. The following sequence, beginning with a cyclic…
A: (1) Ring opening of A (Hemiocetal):
Q: can be 4. (a) The following compound synthesised via Robinson annulation. Provide retrosynthetic…
A: Robinson annulation is a reaction which uses a ketone and a methyl vinyl ketone (α,β-unsaturated…
Q: Below is a schematic representation of possible reaction that compound X can undergo.Use the scheme…
A: a. The IUPAC name for Compound X is
Q: Bhs tổ syhthesize the target compound below from the indicated starting materials.You may use any…
A: Organic reaction mechanisms
Q: 3. Write a detailed, stepwise mechanism for the following reaction. CH3 + Cl2 -CH2CI + HC1
A: We'll answer the first question since the exact one wasn't specified. Please submit a new question…
Q: an efficient and plausible synthesis for the following transformation. (use common Suggest reagents,…
A:
Q: PROPOSE A POSSIBLE SYNTHESIS FOR THE GIVEN TRANSFORMATION
A:
Q: と3 (は)
A:
Q: Fluorination of a benzene ring can be accomplished with Selectfluor, a reagent that contains a…
A: The fluorination of benzene ring requires the application of certain fluorinating agents.…
Q: Devise a stepwise mechanism for the following reaction, a key step in the synthesis of the…
A: The diester undergoes Dieckmann reaction in the presence of base to form beta-ketoester. The base…
Q: Fluorination of a benzene ring can be accomplished with Selectfluor, a reagent that contains a…
A: Electrophilic-aromatic substitution comprises attack of benzene's pi bond on existing electrophile…
Q: Write a complete stepwise mechanism for the following reaction. Show all electron flow with arrows…
A: Given Reaction of Benzoyl chloride with 2 equivalent of Ethylmagnesiumbromide (Grignard reagent)…
Q: 10. Provide a detailed, stepwise mechanism for the following reaction, clearly depicting proper…
A: Mechanism = To be determined
Q: The compound below can undergo E2 reaction mechanism upon addition of sodium chloride. F Select one:…
A: Elimination reaction of alkyl halide: Alkyl halide gives an elimination reaction by reacting with a…
Q: 1. Provide necessary reagents to perform following FG transformations: NH iBu- -Br iBu NH2 NH2 NH2…
A: In this question, we have to find out the correct answer of given problem by the help of the…
Q: Advanced Electron Pushing!! Provide a detailed, stepwise mechanism for the following…
A: NaOMe is very strong base so it take acidic hydrogen nearest to the carbonyl group.
Q: Consider the following transformation below, which Prof. Ingoglia conducted during his post-doctoral…
A:
Q: 2. Propose a reasonable synthesis of compound 2 from compound 1. Use no more than 4 steps. Он он он…
A: "Ardnt-Eistert Homologation" is mainly used for the conversion of a carboxylic acid to larger…
Q: Outline a retrosynthetic analysis to show how the following multi-step synthesis can bè čáfrled dut…
A:
Q: Propose a feasable synthesis of the target molecule (TM, Fig. 1) from the indicated starting…
A: This is the concept of reaction mechanism
Q: 3. Draw a detailed reaction coordinate diagram. 4. Provide transition state drawings for each step…
A: The chemical reaction involves the combination of two or more reactants in an appropriate molar…
Q: C. Using any necessary reagents of 2 carbons or less, and any other necessary inorganic reagents,…
A: The synthesis of the given compound is given below.
Q: 9. Provide a stepwise synthesis for the following
A:
Q: 7. Propose an efficient synthesis of for each of the following transformation: a) b)
A:
Q: (−)-Hyoscyamine, an optically active drug used to treat gastrointestinal disorders, is isolated from…
A: (a) (-)-Hyoscyamine contains two hydrogens which are acidic, which are benzylic and alcoholic…
Q: 1) Compare and contrast the similarities and differences between the mechanisms of the carbonyl…
A: According to Q&A guidelines of Bartleby, we are supposed to answer only the first 3 sub-parts of…
Q: Part 6. Synthesis. Provide the reagents necessary to accomplish the following multistep syntheses…
A: Applying concept of organic synthesis and name reaction.
Q: Explain why electrophilic aromatic substitution in Pyrrole takes place at C-2 positions whereas, in…
A: Note: according to our guidelines we are supposed to answer only one question
Q: Provide a plausible arrow pushing mechanism for the reaction below. cat. H-A MeO MeOH
A: Organic reaction mechanisms
Q: 10. Provide a detailed, stepwise mechanism for the following reaction, clearly depicting proper…
A: This reaction is intramolecular Claisen condensation reaction. Where enolate reacts with ester to…
Q: Q6. Provide a step-wise synthesis for the following compound using benzene as a starting material.…
A:
Q: Answer ALL parts a) - c). In the following reaction sequence, the synthesis of compound 4 is…
A: The solution is given below:
Q: Write an efficient synthesis for the following transformation
A: As per guidelines if multipart questions posted , first question is allowed to answer, please repost…
Q: Propose an efficient synthesis of the following molecules. I.
A: 1- First we need to identify the starting material so we will do retro-synthesis for this given…
Q: (i). Draw a step wise mechanism for the synthesis of chlorobenzene using Benzene as the starting…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- A key step in the synthesis of β-vetivone, a major constituent of vetiver, a perennial grass found in tropical and subtropical regions of the world, involved the reaction of compound A and dihalide B with two equivalents of LDA to form C. Draw a stepwise mechanism for this reaction. β-Vetivone contains a spiro ring system—that is, two rings that share a single carbon atom.Below is a schematic representation of possible reactions that Compound X can undergo. Use the scheme to answer the following questionsA)What is the IUPAC name for Compound X?B)What type of reaction (s) is/are represented by (i) and (ii)?C)Compound X undergo transitions through either [A] or [B] to produce compounds [1], [2], [3] and [4]. Draw the structures of [A] and [B]. D)Illustrating with reaction mechanisms, show how compounds [1], [2], [3] and [4] are formed.E)Which of the compounds in the following pairs will occur in relatively higher yields and why?I)[1] and [2] II)[3] and [4]1. Demonstrate how to convert toluene to the compounds via stepwise mechanism.
- (−)-Hyoscyamine, an optically active drug used to treat gastrointestinal disorders, is isolated from Atropa belladonna, the deadly nightshade plant, by a basic aqueous extraction procedure. If too much base is used during isolation, optically inactive material is isolated. (a) Explain this result by drawing a stepwise mechanism. (b) Explain why littorine, an isomer isolated from the tailflower plant in Australia, can be obtained optically pure regardless of the amount of base used during isolation.Below is a schematic representation of possible reactions that Compound X can undergo. Use the scheme to answer the following questions. A. What is the IUPAC name for Compound X? B. What type of reaction (s) is/are represented by (i) and (ii)? C. Compound X undergo transitions through either [A] or [B] to produce compounds [1], [2], [3] and [4]. Draw the structures of [A] and [B]. D. Illustrating with reaction mechanisms, show how compounds [1], [2], [3] and [4] are formed. E. Which of the compounds in the following pairs will occur in relatively higher yields and why? [1] and [2] [3] and [4] The attached image contains the scheme.Below is a schematic representation of possible reactions that Compound X can undergo. Use the scheme to answer the following questions. –a. What is the IUPAC name for Compound X? b. What type of reaction (s) is/are represented by (i) and (ii)? c. Compound X undergo transitions through either [A] or [B] to produce compounds [1], [2], [3] and [4]. Draw the structures of [A] and [B]. d. Illustrating with reaction mechanisms, show how compounds [1], [2], [3] and [4] are formed.e. Which of the compounds in the following pairs will occur in relatively higher yields and why?i. [1] and [2] ii. [3] and [4]
- The sex pheromone (matsuone) of a parasitic insect (Matsucoccus) that infests pine trees was prepared in a multistep synthesis from (−)-citronellol by way of the nitrile shown.(a) Relate the nitrile to (−)-citronellol by a retrosynthetic analysis. (b) Convert your retrosynthesis to a synthesis, showing appropriate reagents for each step.(-)-Hyoscyamine, an optically active drug used to treat gastrointestinal disorders, is isolated from atropabelladonna, the deadly nightshade plant, by a basic aqueous extraction procedure. If too much base is used during isolation, optically inactive material is isolated A) explain this result by drawing a stepwise mechanismplease help with this question. thank you. The following sequence, beginning with a cyclic hemiacetal (compound A), was part of a recently reported enantiospecific synthesis of a powerful sex pheromone (currently used in pest management) of the mealybug Pseudococcus viburni: Draw the structures of compound B and C. Provide a plausible mechanism to explain the transformation from compound C into compound D. Identify the reagents you would need to convert compound D into compound F (in just two steps). Also identify the structure of compound E.
- Do not give handwriting solution. please draw out what compound A is and then provide aroow pushing mechanism for thisreaction! thanks01 Cive the ctrientiono of Comnand 4 2.2 Provide a reasonable arrowpushing mechanism for the reaction in 2.1Using any necessary organic and inorganic reagents, show how you can carry out the chemical conversions shwon below. Please answer parts a, b, and c.Tamoxifen is an estrogen receptor modulator that is used in the treatment of breast cancer. Provide the missing reagents and the structure of compound A in the synthesis of tamoxifen.

