Chapter9: Alkynes: An Introduction To Organic Synthesis
Section9.SE: Something Extra
Problem 21MP
Related questions
Question
The following two isomeric
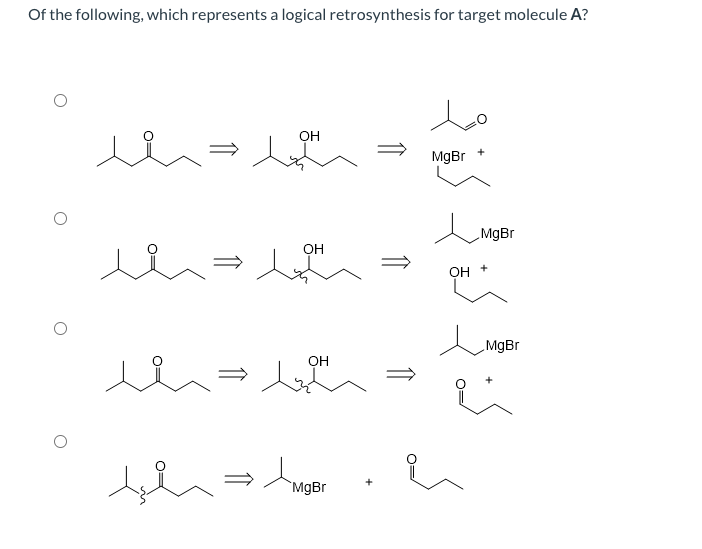
Transcribed Image Text:Of the following, which represents a logical retrosynthesis for target molecule A?
Lo
он
MgBr
MgBr
OH
OH
MgBr
OH
`MgBr
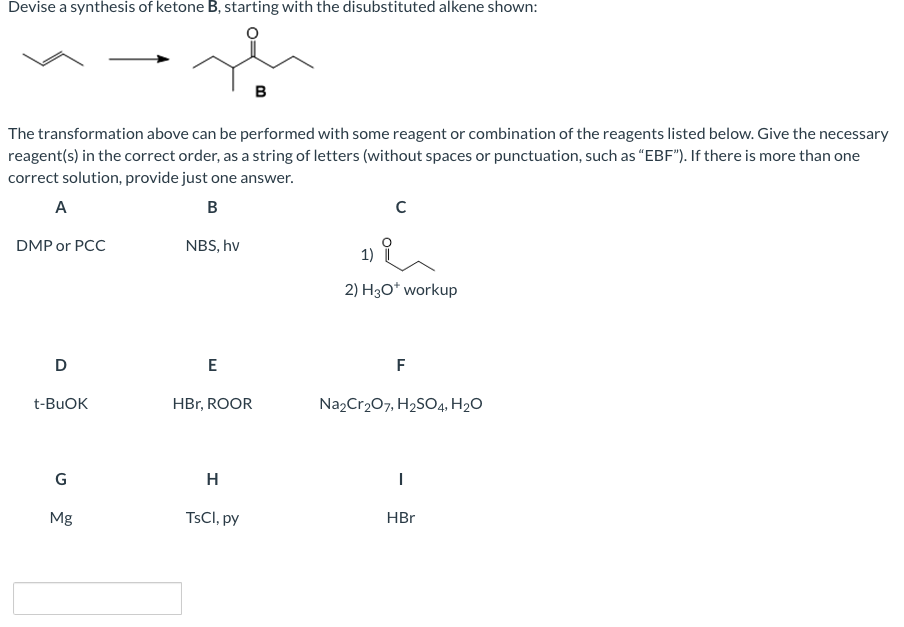
Transcribed Image Text:Devise a synthesis of ketone B, starting with the disubstituted alkene shown:
в
The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary
reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one
correct solution, provide just one answer.
А
B
DMP or PCC
NBS, hv
2) H30* workup
D
E
F
t-BUOK
HBr, ROOR
Na,Cr207, H2SO4, H2O
G
H
Mg
TSCI, py
HBr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
For step 4 synthesis can you explain what each reagent does in the synthesis? Like, expand on if it's an addition reaction or substitution or elimination and what type, etc.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning