energy level atomic number d-block orbital/sublevel family period f-block s-block p-block electrons is one of the bases of how are the with Increasing elements in the Periodic Table are arranged. Elements similar properties are grouped as, 2 where the elements have the same number of electrons in their outermost shell and 3, where the elements have the same number of main energy levels. 1 The periodic table of elements consists of columns (Group 1A and Group 2A); the (Groups 3A to 8A); the, 2B); and the 4 6 columns 10 columns (Groups 3B to 14 columns under the group of 2 6 lanthanides and actinides. This number of columns for each block corresponds to the number of electrons capable of filling up the s, p, dand forbitals. Given the electron configuration of the element oxygen, 1s2 s2$ 2p4, the numerical coefficient is the 8_, the letter is 9 the and the superscript is the number of the sublevel. 10_ in
energy level atomic number d-block orbital/sublevel family period f-block s-block p-block electrons is one of the bases of how are the with Increasing elements in the Periodic Table are arranged. Elements similar properties are grouped as, 2 where the elements have the same number of electrons in their outermost shell and 3, where the elements have the same number of main energy levels. 1 The periodic table of elements consists of columns (Group 1A and Group 2A); the (Groups 3A to 8A); the, 2B); and the 4 6 columns 10 columns (Groups 3B to 14 columns under the group of 2 6 lanthanides and actinides. This number of columns for each block corresponds to the number of electrons capable of filling up the s, p, dand forbitals. Given the electron configuration of the element oxygen, 1s2 s2$ 2p4, the numerical coefficient is the 8_, the letter is 9 the and the superscript is the number of the sublevel. 10_ in
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter5: Atomic Theory : The Nuclear Model Of The Atom
Section: Chapter Questions
Problem 62E
Related questions
Question
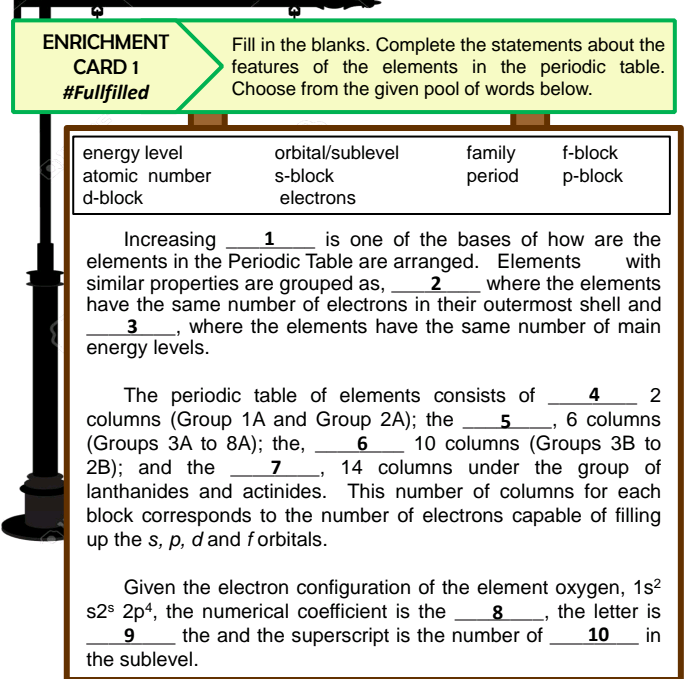
Transcribed Image Text:ENRICHMENT
Fill in the blanks. Complete the statements about the
features of the elements in the periodic table.
Choose from the given pool of words below.
CARD 1
#Fullfilled
energy level
atomic number
orbital/sublevel
family
period
f-block
s-block
p-block
d-block
electrons
is one of the bases of how are the
with
Increasing
elements in the Periodic Table are arranged. Elements
similar properties are grouped as, 2 where the elements
have the same number of electrons in their outermost shell and
1
3, where the elements have the same number of main
energy levels.
The periodic table of elements consists of 4
columns (Group 1A and Group 2A); the
(Groups 3A to 8A); the,
2B); and the
lanthanides and actinides. This number of columns for each
2
_, 6 columns
10 columns (Groups 3B to
14 columns under the group of
7
block corresponds to the number of electrons capable of filling
up the s, p, dand forbitals.
Given the electron configuration of the element oxygen, 1s2
s2$ 2p4, the numerical coefficient is the 8, the letter is
the and the superscript is the number of
10
in
the sublevel.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning