As one proceeds from left to right across Period 3 of the Periodic Table, there is a decrease m! a) ionization energy b) electron affinity c) metallic characteristics d) valence electrons Which of the following is a synthetic element? a) Americium b) Uranium c) Fermium d) All of the above e) Only a and c What does the graph to the right best represent? a) atomic radii of Period 2 b) ionization energy in Period 3 c) atomic radii in the Chalcogen group d) Electronegativity in Group 17 e) Who in the world knows!! INCREASING ATOMIC # ->
As one proceeds from left to right across Period 3 of the Periodic Table, there is a decrease m! a) ionization energy b) electron affinity c) metallic characteristics d) valence electrons Which of the following is a synthetic element? a) Americium b) Uranium c) Fermium d) All of the above e) Only a and c What does the graph to the right best represent? a) atomic radii of Period 2 b) ionization energy in Period 3 c) atomic radii in the Chalcogen group d) Electronegativity in Group 17 e) Who in the world knows!! INCREASING ATOMIC # ->
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 158CWP
Related questions
Question
Please answer all parts
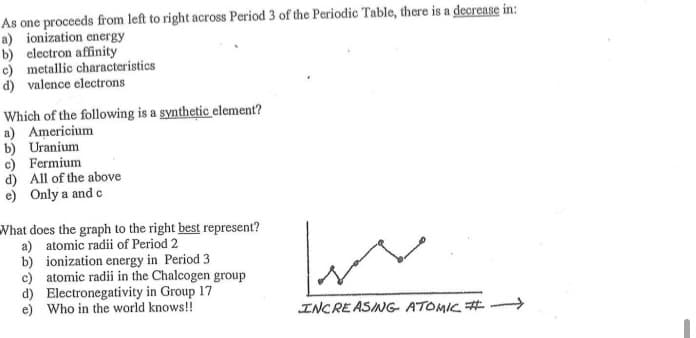
Transcribed Image Text:As one proceeds from left to right across Period 3 of the Periodic Table, there is a decrease in:
a) ionization energy
b) electron affinity
c) metallic characteristics
d) valence electrons
Which of the following is a synthetic element?
a) Americium
b) Uranium
c) Fermium
d) All of the above
e) Only a and c
What does the graph to the right best represent?
a) atomic radii of Period 2
b) ionization energy in Period 3
c) atomic radii in the Chalcogen group
d) Electronegativity in Group 17
e) Who in the world knows!!
INCRE ASING- ATOMIC #
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning