ent # 4 (ch. 15) i Saved Help 5 attempts left Check my work Be sure to answer all parts. Enter your answers in scientific notation. The following reactions have the indicated equilibrium constants at a particular temperature: N2(g) + O2(g) = 2NO(g) K = 4.3 x 10-25 2NO(g) + 02(g) = 2NO,(g) K = 6.4 x 109 Determine the values of the equilibrium constants for the following equations at the same temperature: (a) 4NO(g) = N2g) + 2NO2(g) x 10 (b) 4NO2(g) = 2N2(g) + 402(g) x 10 (c) 2NO(g) + 2N02(g) = 30,(g) + 2N2(g) x 10 < Prev 7 of 15 Next > FEB 25 w/
ent # 4 (ch. 15) i Saved Help 5 attempts left Check my work Be sure to answer all parts. Enter your answers in scientific notation. The following reactions have the indicated equilibrium constants at a particular temperature: N2(g) + O2(g) = 2NO(g) K = 4.3 x 10-25 2NO(g) + 02(g) = 2NO,(g) K = 6.4 x 109 Determine the values of the equilibrium constants for the following equations at the same temperature: (a) 4NO(g) = N2g) + 2NO2(g) x 10 (b) 4NO2(g) = 2N2(g) + 402(g) x 10 (c) 2NO(g) + 2N02(g) = 30,(g) + 2N2(g) x 10 < Prev 7 of 15 Next > FEB 25 w/
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 20QAP: Consider the following hypothetical reactions and their equilibrium constants at 75C,...
Related questions
Question
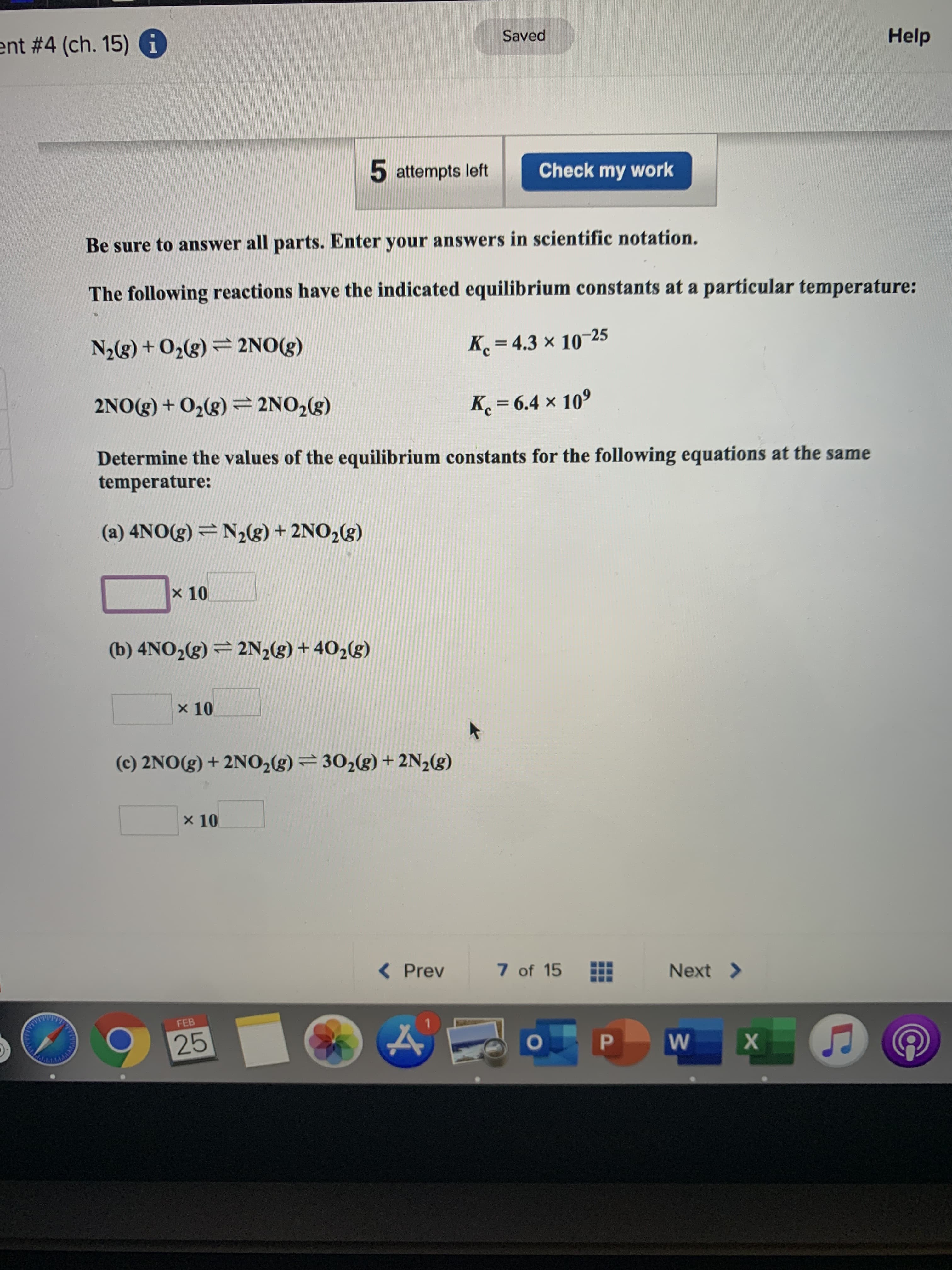
Transcribed Image Text:ent # 4 (ch. 15) i
Saved
Help
5 attempts left
Check my work
Be sure to answer all parts. Enter your answers in scientific notation.
The following reactions have the indicated equilibrium constants at a particular temperature:
N2(g) + O2(g) = 2NO(g)
K = 4.3 x 10-25
2NO(g) + 02(g) = 2NO,(g)
K = 6.4 x 109
Determine the values of the equilibrium constants for the following equations at the same
temperature:
(a) 4NO(g) = N2g) + 2NO2(g)
x 10
(b) 4NO2(g) = 2N2(g) + 402(g)
x 10
(c) 2NO(g) + 2N02(g) = 30,(g) + 2N2(g)
x 10
< Prev
7 of 15
Next >
FEB
25
w/
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning